��Ŀ����
����Ŀ���о�+6�۸��β�ͬ��������������ʽ�������ԣ�ijС��ͬѧ��������ʵ�飺
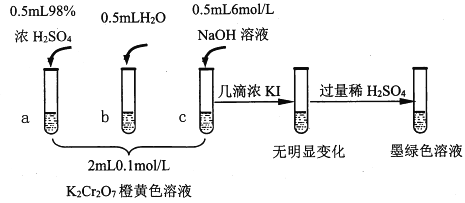
��֪��Cr2O72��(��ɫ)+H2O![]() 2CrO42��(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
2CrO42��(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
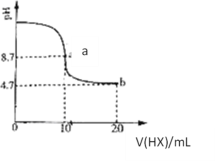
��1���Թ�c��b�Աȣ��Ʋ��Թ�c��������_____________________��
��2���Թ�a��b�Աȣ�a����Һ��ɫ�������Ϊ�¶�Ҳ��Ӱ��ƽ����ƶ�����ɫ���һ����c(H+)����Ӱ��Ľ��������Ϊ��ɫ����һ����c(H+)�����ƽ���Ӱ�졣����Ϊ�Ƿ���Ҫ�����ʵ��֤����________�����ǡ�����������______��
��3���Թ�c�����μ�KI��Һ������ϡH2SO4��������ͼ��ʵ�����ó��Ľ�����________��
��4��С��ͬѧ�õ�ⷨ������Cr2O72����ˮ��̽����ͬ���ضԺ�Cr2O72����ˮ������Ӱ�죬������±���ʾ��Cr2O72������ʼŨ�ȣ��������ѹ�����ʱ�����ͬ����
ʵ�� | �� | �� | �� | �� |
�Ƿ����Fe2(SO4)3 | �� | �� | ����5g | �� |
�Ƿ����H2SO4 | �� | ����1mL | ����1mL | ����1mL |
�缫���� | ����������Ϊʯī | ����������Ϊʯī | ����������Ϊʯī | ����Ϊʯī����Ϊ�� |
Cr2O72����ȥ����/% | 0.922 | 12.7 | 20.8 | 57.3 |
�ٶԱ�ʵ�颡��ʵ�颢��֪��_________�������ߡ����͡���pH�������Cr2O72����ȥ���ʡ�
��ʵ�颢��Cr2O72���ŵ�ĵ缫��ӦʽΪ___________________________________��
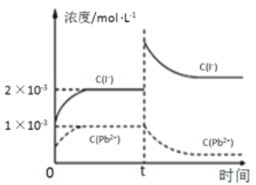
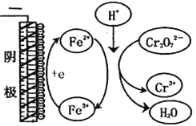
��ʵ�颣��Fe3+ȥ��Cr2O72���Ļ�����ͼ��ʾ����ϴ˻���������ʵ��iv��Cr2O72��ȥ������߽϶��ԭ����_______________ ��
���𰸡���Һ���ɫ �� Cr2O72��(��ɫ)+H2O![]() 2CrO42��(��ɫ)+2H+������Ϊ���ȷ�Ӧ������Ũ��������ˮ���¶����ߣ�ƽ�������ƶ�����ҺӦ��Ϊ��ɫ������Һ��ɫ���˵����c(H+)����Ӱ��ƽ��Ľ�� ���������£� CrO42����������I�������������£� Cr2O72����������I�� ���� Cr2O72��+6e��+ 14H+=2 Cr3++7 H2O ����Feʧ��������Fe2+��Fe2+��Cr2O72���������������·�Ӧ����Fe3+��Fe3+�������õ�������Fe2+��������ԭCr2O72����Fe2+��ѭ�����������Cr2O72����ȥ����
2CrO42��(��ɫ)+2H+������Ϊ���ȷ�Ӧ������Ũ��������ˮ���¶����ߣ�ƽ�������ƶ�����ҺӦ��Ϊ��ɫ������Һ��ɫ���˵����c(H+)����Ӱ��ƽ��Ľ�� ���������£� CrO42����������I�������������£� Cr2O72����������I�� ���� Cr2O72��+6e��+ 14H+=2 Cr3++7 H2O ����Feʧ��������Fe2+��Fe2+��Cr2O72���������������·�Ӧ����Fe3+��Fe3+�������õ�������Fe2+��������ԭCr2O72����Fe2+��ѭ�����������Cr2O72����ȥ����
��������
��1������Cr2O72��(��ɫ)+H2O![]() 2CrO42��(��ɫ)+2H+���Թ�c�м���NaOH��Һ������H������ʹƽ��������Ӧ������У���Һ��ɫ�ɳ�ɫ��Ϊ��ɫ��
2CrO42��(��ɫ)+2H+���Թ�c�м���NaOH��Һ������H������ʹƽ��������Ӧ������У���Һ��ɫ�ɳ�ɫ��Ϊ��ɫ��
��2��Cr2O72��(��ɫ)+H2O![]() 2CrO42��(��ɫ)+2H+ ��H= +13.8 kJ/mol���÷�ӦΪ���ȷ�Ӧ���Թ�a�м���Ũ���ᣬŨ������ˮ�ų��������¶����ߣ�ƽ��Ӧ�����ƶ�����Һ�Ի�ɫ����ʵ�ʵ�ʵ����������Һ��ɫ���˵��c(Cr2O42��)���࣬ƽ�����淴Ӧ������У���˸�ʵ������Ӧ��c(H��)��ƽ�������ƶ��Ľ����������Ҫ�����ʵ��֤����
2CrO42��(��ɫ)+2H+ ��H= +13.8 kJ/mol���÷�ӦΪ���ȷ�Ӧ���Թ�a�м���Ũ���ᣬŨ������ˮ�ų��������¶����ߣ�ƽ��Ӧ�����ƶ�����Һ�Ի�ɫ����ʵ�ʵ�ʵ����������Һ��ɫ���˵��c(Cr2O42��)���࣬ƽ�����淴Ӧ������У���˸�ʵ������Ӧ��c(H��)��ƽ�������ƶ��Ľ����������Ҫ�����ʵ��֤����
��3������ʵ�������������£�CrO42����������I��������������������������£�Cr2O72��������I��������ԭΪCr3������Һ��Ϊī��ɫ�����������ӷ�Ӧ����ʽΪ6I����Cr2O72����14H��=3I2��2Cr3����7H2O����˵ó�����Ϊ���������£� CrO42����������I�������������£� Cr2O72����������I�� ��
��4����ʵ��i��ʵ��ii��ͬ����ʵ��ii����1mLH2SO4��pH���ͣ�ȥ�������ߣ�������pH�������Cr2O72����ȥ���ʣ�
��ʵ��ii��Cr2O72��Ӧ�������Ϸŵ磬�õ��ӱ���ԭ��Cr3��������ʻ���Ϊ���ԣ����Cr2O72���ŵ�ķ�ӦʽΪCr2O72����14H����6e��=2Cr3����7H2O��
�۸���ͼʾ��ʵ��iv����������������ʧȥ��������Fe2����Fe2����Cr2O72�������������·�Ӧ����Fe3����Fe3���������õ�������Fe2����������ԭCr2O72����Fe2��ѭ�����������Cr2O72����ȥ���ʡ�