��Ŀ����
����Ŀ��(1)250��ʱ�������Ͻ�Ϊ���������ʼ���Ϊ 8L ���ܱ�������ͨ�� 5molCO2��3molCH4���ں�ѹ�����·�����Ӧ��CO2(g) +CH4(g)2CO(g) + 2H2(g)��ƽ����ϵ�м�������ʵ����仯������
��Ӧʱ��(s) | 2 | 4 | 6 | 8 |
CH4���ʵ���(mol) | 2.7 | 2.4 | 2.0 | 2.0 |
���¶��£��÷�Ӧ��ƽ�ⳣ��K =_____��
(2)�����Ѵ���������ķ�Ӧ���̣���Ҫ�����¼�����Ӧ(����Ϊ 25����1.01��105Pa �ⶨ)
�� CH3OCH3(g) + H2O(l)2CH3OH(l) ��H����24.52kJ/mol
�� CH3OH(l) + H2O(l)CO2(g) + 3H2(g) ��H����49.01kJ/mol
�� CO(g) + H2O(l)CO2(g) + H2(g) ��H����41.17kJ/mol
�� CH3OH(l)CO (g) + 2H2(g) ��H����90. 18kJ/mol
�� CH3OCH3(g) +3H2O(l)2CO2(g) + 6H2(g) ��H =_____kJ/mol
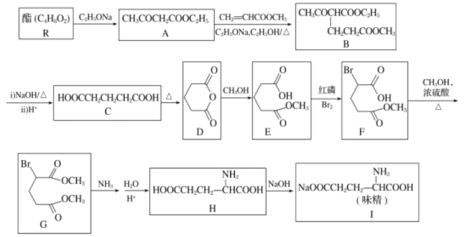
(3)����(2)�ж����Ѵ���������Ĺ����в�ò�ͬ�¶��¸������������������ѵ�ת���ʹ�ϵ��ͼ��ʾ��
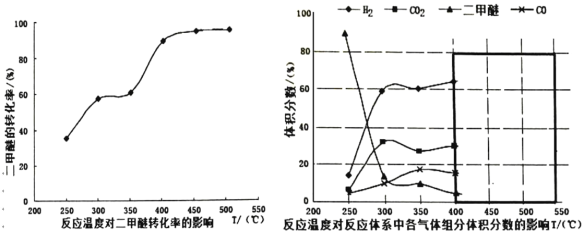
������Ϊ��Ӧ���Ƶ�����¶�ӦΪ_____________��������__________��
A.300��350�� B.350��400�� C.400��450�� D.450��500��
����һ�����Ⱥ��ݵ��ܱ������У�����һ�����ļ״����ʽ����ƽ�⣬���¿�����Ϊ�÷�Ӧ�ﵽƽ��״̬���ж����ݵ���____________��
A.��ϵ���¶Ȳ��ٸı� B.�����ƽ����Է����������ֲ���
C.CO ������������� D.������ܶȱ��ֲ���
�� ���¶ȴﵽ 400���Ժ������� CO2�Լ�����ͬ�ı仯�������Խ��ͣ��� CO �� H2���������Ҳ�Լ�����ͬ�ı仯�������ߡ����ʱ�����ķ�ӦΪ_______________����ͼ�к�ɫ�����ڻ��� 400���Ժ�� CH3OCH3�� CO ��������ı仯����____��
���𰸡�0.02 +122.54 C �����ѵ�ת���ʸߣ��Ҳ���CO2��H2����������ϴ� AD CH3OCH3(g)+CO2(g)![]() 3CO(g)+3H2(g)
3CO(g)+3H2(g) 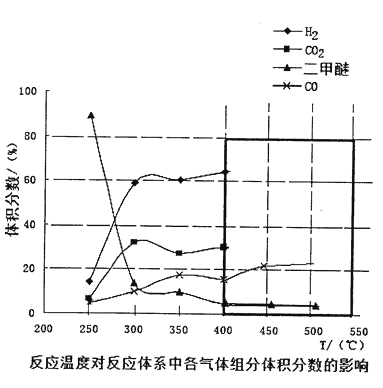
��������
��1�����ݷ�Ӧ�и�����Ũ�ȱ仯��֮�ȵ����仯ѧ������֮�����������ƽ��ʱ��Ũ�ȣ�Ȼ�����ƽ�ⳣ���������ƽ�ⳣ����
��2�����ø�˹���ɽ��
��3���ٸ��ݷ�Ӧ���������Խ�ͣ���ת����Խ�ߣ��������������Խ�ߣ��䷴Ӧѡ����Խ�ý��з�����
�ڸ���ƽ��״̬�¸��������ٱ仯���з�����
�۶������� CO2�Լ�����ͬ�ı仯�������Խ��ͣ��� CO �� H2���������Ҳ�Լ�����ͬ�ı仯�������߿�ȷ����Ӧ�������������ԭ���غ���ƽ�����ݸ����ʵı仯���ƺͷ�Ӧ�л�ѧ��������ϵȷ�����߱仯��
��1���ɱ������ݿ�֪����Ӧ�ﵽƽ���n(CH4)=2mol����n(CH4)=3mol-2mol=1mol����ƽ��ʱn(CO2)=4mol��n(CO)=2mol��n(H2)=2mol����ʼ���Ϊ8L����ƽ��ʱ���ΪV����![]() ����V=10L���÷�Ӧ��ƽ�ⳣ��
����V=10L���÷�Ӧ��ƽ�ⳣ�� ��
��
��2����֪���� CH3OCH3(g)+H2O(l)![]() 2CH3OH(l)��H=+24.52kJ/mol
2CH3OH(l)��H=+24.52kJ/mol
�� CH3OH(l)+H2O(l)![]() CO2(g)+3H2(g)��H=+49.01kJ/mol
CO2(g)+3H2(g)��H=+49.01kJ/mol
�� CO(g)+H2O(l)![]() CO2(g)+H2(g)��H=-41.17kJ/mol
CO2(g)+H2(g)��H=-41.17kJ/mol
�� CH3OH(l)![]() CO(g)+2H2(g)��H=+90. 18kJ/mol
CO(g)+2H2(g)��H=+90. 18kJ/mol
���ݸ�˹���ɢ�+��+��+�ܿɵã�CH3OCH3(g)+3H2O(l)![]() 2CO2(g)+6H2(g)��H=+122.54kJ/mol��
2CO2(g)+6H2(g)��H=+122.54kJ/mol��
��3���ٸ���ͼ��֪��400450��ʱ�������ѵ�ת���ʸߣ��Ҳ���CO2��H2����������ϴʴ�Ϊ��C�������ѵ�ת���ʸߣ��Ҳ���CO2��H2����������ϴ�
��A����Ӧ��Ϊ���ȷ�Ӧ��װ��Ϊ���ȣ�����ϵ�¶Ȼή�ͣ�ƽ��ʱ����ϵ���¶Ȳ��ٸı䣬��A�������⣻
B��װ���е�����ʼ��Ϊ1��2��CO��H2���������ƽ����Է�������ʼ�ձ��ֲ��䣬����˵����ѧƽ��״̬����B���������⣻
C��װ���е�����ʼ��Ϊ1��2��CO��H2����CO���������ʼ�ղ��䣬����˵����ѧƽ��״̬����C���������⣻
D������![]() �����������������V���䣬��������ܶȱ��ֲ���˵����Ӧ�ﵽƽ��״̬����D�������⣻
�����������������V���䣬��������ܶȱ��ֲ���˵����Ӧ�ﵽƽ��״̬����D�������⣻
�ʴ�Ϊ��AD��
�����¶ȴﵽ400���Ժ������� CO2�Լ�����ͬ�ı仯�������Խ��ͣ�˵���������� CO2������Ӧ��Ҷ���ϵ��Ϊ1:1���� CO �� H2���������Ҳ�Լ�����ͬ�ı仯�������ߣ�˵��CO��H2��������Ҷ���ϵ��Ϊ1:1������ԭ���غ��֪��������Ӧ�� CH3OCH3(g)+CO2(g)![]() 3CO(g)+3H2(g)����CH3OCH3���٣��������½���CO���࣬�������������ұ仯����Ϊ1:3����ͼ���������Ϊ��
3CO(g)+3H2(g)����CH3OCH3���٣��������½���CO���࣬�������������ұ仯����Ϊ1:3����ͼ���������Ϊ�� ��
��

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д�����Ŀ������ʵ��������ʵ���������ƥ�����
ʵ����� | ʵ������ | |
A | ��ʢ�и������������Һ���Թ���ͨ����������ϩ���� | ��Һ����ɫ����ȥ�����ú���Һ�ֲ� |
B | ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в���Ũ�̲��к�ɫ�������� |
C | ��ʢ�б��������������Һ���Թ��еμ�ϡ���� | �д̼�����ζ�����������Һ����� |
D | ��ʢ��FeCl3��Һ���Թ��мӹ������ۣ�������1��KSCN��Һ | ��ɫ����ʧ����KSCN����Һ��ɫ���� |
A. AB. BC. CD. D
����Ŀ����������ʵ���ó�����Ӧ������ȷ����
ʵ����ʵ | ���� | |
A | ����ͬ�¶��£���1 mL0.2 mol/LNaOH��Һ�е���2��0.1 mol/LMgCl2��Һ��������ɫ�������ٵμ�2��0.1 mol/LFeCl3��Һ�������ɺ��ɫ���� | �ܽ�ȣ�Mg(OH)2>Fe(OH)3 |
B | ij������ʹʪ�����ɫʯ����ֽ��� | ������ˮ��Һһ���Լ��� |
C | ͬ��ͬѹ�£������pH=3��HA��HB������ֱ���������п��Ӧ����ˮ���ռ����壬HA�ų����������ҷ�Ӧ���ʿ� | HB�����Ա�HAǿ |
D | SiO2����������ᷴӦ������Ӧ | SiO2������������ |
A.AB.BC.CD.D