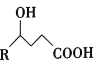
��Ŀ����
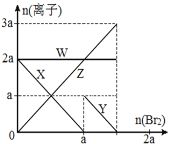
����Ŀ�������ԣ�Br2 > Fe3+> I2���� a mol FeI2 �� a mol BaCl2 �Ļ����Һ�л���ͨ�� b mol Br2�������Ͻ��裬��Һ�����ӵ����ʵ����� Br2����ı仯��ͼ��ʾ(����������ˮ�⡢ˮ�ĵ��뼰��Һ����仯)��������˵������ȷ����( )
A.���ӵĻ�ԭ�� I-> Fe2+> Br-
B.����������Һ�����ӵĶ�Ӧ��ϵ�ǣ� X-Fe2+��Y-I-��Z-Br-��W-Cl-
C.��4a =3bʱ����Ӧ�������Ũ�ȱȣ�c(Fe2+):c(Fe3+):c(Br-) = 1:2:8
D.��3a��2bʱ�����������ӷ���ʽ��2Fe2++ 4I-+ 3Br2=2Fe3++ 2I2+6Br-
���𰸡�B
��������
������ԭ��Ӧ�У���������������>��������������ԣ���ԭ���Ļ�ԭ��>��ԭ����Ļ�ԭ�ԣ���������������ԭ��ǿ�����ӣ���������ԭ���������ӣ���amolFeI2��amolBaCl2�Ļ����Һ�л���ͨ��bmolBr2����֪�����ԣ�Br2>Fe3+>I2�������ӵĻ�ԭ��I->Fe2+>Br-���������ȱ�������������������ӣ���ͨ��Br2����Ϊ0��amol�Ĺ����У������Ӵ����������0��ͨ��Br2����Ϊa��bmol�Ĺ����У��������Ӵ�amol����0������������������Br-�ӿ�ʼ��������Cl-һֱ���䣬�ݴ˻ش��жϡ�
A�������ԣ�Br2>Fe3+>I2�������ӵĻ�ԭ��I->Fe2+>Br-����A���������⣻
B���������Ϸ�����Clһֱ���伴WΪCl��Br�ӿ�ʼ��������ZΪBr���������ȱ����������Կ�ʼ��С��ΪI��XΪI����������������ӣ�����YΪFe2+����B�������⣻
C����4a=3bʱ����2I+Br2=I2+2Br��֪��amolBr2����2amolI������2amolBr������2Fe2++Br2=2Fe3++2Br��֪��![]() amolBr2����
amolBr2����![]() amolFe2+������
amolFe2+������![]() amolFe3+��
amolFe3+��![]() amolBr��ʣ��
amolBr��ʣ��![]() amolFe2+�����Է�Ӧ�������Ũ�ȱȣ�c(Fe2+)��c(Fe3+)��c(Br)=1:2:8����C���������⣻
amolFe2+�����Է�Ӧ�������Ũ�ȱȣ�c(Fe2+)��c(Fe3+)��c(Br)=1:2:8����C���������⣻
D����3a2bʱ��������ӡ���������ȫ�������������������ӷ���ʽΪ��2Fe2++4I+3Br2�T2Fe3++2I2+6Br����D���������⣻
�ʴ�Ϊ��B��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�����Ŀ����Դ������������������Դ���õ��ǵ����������Ż��⡣�������ѧ��ѧ֪ʶ�ش��������⣺
��1�������ϰ�װ��ת��������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
��֪��N2(g) + O2(g)��2NO(g) ��H��+180.5 kJ �� mol��1��
2C(s)+ O2(g)��2CO(g) ��H����221.0 kJ �� mol��1��
C(s)+ O2(g)��CO2(g) ��H����393.5 kJ �� mol��1��
��β��ת����Ӧ2NO(g) +2CO(g)��N2(g)+2CO2(g)����H��________________��
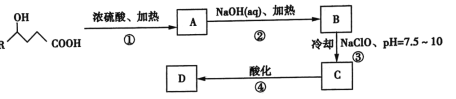
��2������β�������Ƕ�CO�ĺ�����������ȼ�ϵ��Ϊ����ԭ������װ������ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ�������������ƶ���

����˵������ȷ����_____________(����ĸ���)��
A�������ĵ缫��ӦʽΪ��CO + O2���D2e����CO2
B������ʱ�����ɵ缫aͨ������������缫b
C������ʱ�缫b��������O2���ɵ缫aͨ�����������缫bǨ��
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
��3��ij���᳧���ü״�������ˮ����һ�������£����ˮ�м���CH3OH����HNO3��ԭ��N2�����÷�Ӧ����32 g CH3OHת��6 mol���ӣ���μӷ�Ӧ�Ļ�ԭ���������������ʵ���֮��Ϊ______________��
��4��ú�ļ��Һ������ת��ΪCO��H2�����ڴ��������ºϳɼ״�������һ���¶��£���1 L�ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
�� �� | CO | H2 | CH3OH |
Ũ��/(mol��L��1) | 1.2 | 1.0 | 0.6 |
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K��_____________________��
�ڸ�ʱ���ڷ�Ӧ������(H2)��_________________��
��ƽ��ʱCO��ת����Ϊ_________________(����1λС��)��
����Ŀ��(1)250��ʱ�������Ͻ�Ϊ���������ʼ���Ϊ 8L ���ܱ�������ͨ�� 5molCO2��3molCH4���ں�ѹ�����·�����Ӧ��CO2(g) +CH4(g)2CO(g) + 2H2(g)��ƽ����ϵ�м�������ʵ����仯������
��Ӧʱ��(s) | 2 | 4 | 6 | 8 |
CH4���ʵ���(mol) | 2.7 | 2.4 | 2.0 | 2.0 |
���¶��£��÷�Ӧ��ƽ�ⳣ��K =_____��
(2)�����Ѵ���������ķ�Ӧ���̣���Ҫ�����¼�����Ӧ(����Ϊ 25����1.01��105Pa �ⶨ)
�� CH3OCH3(g) + H2O(l)2CH3OH(l) ��H����24.52kJ/mol
�� CH3OH(l) + H2O(l)CO2(g) + 3H2(g) ��H����49.01kJ/mol
�� CO(g) + H2O(l)CO2(g) + H2(g) ��H����41.17kJ/mol
�� CH3OH(l)CO (g) + 2H2(g) ��H����90. 18kJ/mol
�� CH3OCH3(g) +3H2O(l)2CO2(g) + 6H2(g) ��H =_____kJ/mol
(3)����(2)�ж����Ѵ���������Ĺ����в�ò�ͬ�¶��¸������������������ѵ�ת���ʹ�ϵ��ͼ��ʾ��
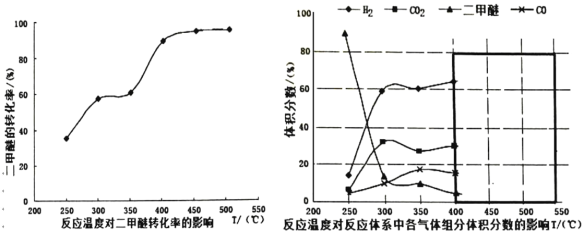
������Ϊ��Ӧ���Ƶ�����¶�ӦΪ_____________��������__________��
A.300��350�� B.350��400�� C.400��450�� D.450��500��
����һ�����Ⱥ��ݵ��ܱ������У�����һ�����ļ״����ʽ����ƽ�⣬���¿�����Ϊ�÷�Ӧ�ﵽƽ��״̬���ж����ݵ���____________��
A.��ϵ���¶Ȳ��ٸı� B.�����ƽ����Է����������ֲ���
C.CO ������������� D.������ܶȱ��ֲ���
�� ���¶ȴﵽ 400���Ժ������� CO2�Լ�����ͬ�ı仯�������Խ��ͣ��� CO �� H2���������Ҳ�Լ�����ͬ�ı仯�������ߡ����ʱ�����ķ�ӦΪ_______________����ͼ�к�ɫ�����ڻ��� 400���Ժ�� CH3OCH3�� CO ��������ı仯����____��