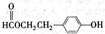��Ŀ����
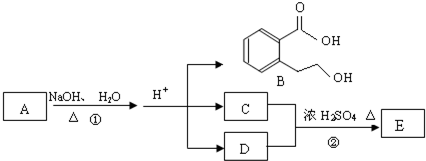
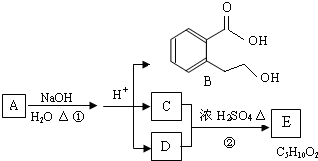
A��B��C��D��E��Ϊ�л����������֮��Ĺ�ϵ��ͼ��ʾ(��ʾ��RCH=CHR'
�����Ը��������Һ�з�Ӧ����RCOOH��R'COOH������R��R'Ϊ���)��
�ش��������⣺
(1)ֱ��������A����Է�������С��90��A������̼����Ԫ�ص�����������Ϊ0.814������Ϊ��Ԫ�أ���A�ķ���ʽΪ_____________��
(2)��֪B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������Ũ����Ĵ��£�B��������C2H5OH������Ӧ�Ļ�ѧ����ʽ��____________________________����Ӧ����Ϊ____________________��
(3)A��������������÷ų���������ʹ������Ȼ�̼��Һ��ɫ����A�Ľṹ��ʽ��__________________
(4)D��ͬ���칹���У�����NaHCO3��Һ��Ӧ�ų�CO2����__________�֣�����Ӧ�Ľṹ��ʽ��__________________��
��1��C5H10O��
(2��HOOC��CH2��COOH��
HOOC��CH2��COOH + 2C2H5OH![]() C2H5OOC��CH2��COOC2H5 +2H2O��������Ӧ����ȡ����Ӧ����
C2H5OOC��CH2��COOC2H5 +2H2O��������Ӧ����ȡ����Ӧ����
(3��HO��CH2��CH2��CH=CH��CH3��
(4��2��CH3CH2CH2COOH��CH3CH(CH3)COOH��
����:
��1��������������Ϊ1��0.814��0.186���ٶ�A����Է�������Ϊ90����N(O)��=1.0463����������ԭ�Ӹ���Ϊ1����A����Է�������Ϊ����86�������෨��86��16/12=5����10����A�÷���ʽΪC5H10O��
(2������B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������B�к���2����COOH�����C��C2H5OH![]() C2H4O2��H2O��֪��CΪCH3COOH����B�к���3��Cԭ�ӣ�����֪B�к���2����COOH����B�л�����һ��CH2������B�Ľṹ��ʽΪHOOC��CH2��COOH��B��������C2H5OH��Ӧ�Ļ�ѧ����ʽΪ��HOOC��CH2��COOH + 2C2H5OH
C2H4O2��H2O��֪��CΪCH3COOH����B�к���3��Cԭ�ӣ�����֪B�к���2����COOH����B�л�����һ��CH2������B�Ľṹ��ʽΪHOOC��CH2��COOH��B��������C2H5OH��Ӧ�Ļ�ѧ����ʽΪ��HOOC��CH2��COOH + 2C2H5OH![]() C2H5OOC��CH2��COOC2H5 +2H2O����Ӧ����Ϊ������Ӧ��
C2H5OOC��CH2��COOC2H5 +2H2O����Ӧ����Ϊ������Ӧ��
(3��A��������������÷ų�������˵��A�к��еĹ�����Ϊ��OH����ʹ������Ȼ�̼��Һ��ɫ��˵��A�к���C=C����AΪֱ�������������ϵ���ʾ�ɵ�A�Ľṹ��ʽΪ��
HO��CH2��CH2��CH=CH��CH3��
(4������NaHCO3��Һ��Ӧ�ų�CO2��˵��Ϊ���ᣬ��ṹ��ʽΪ��CH3CH2CH2COOH��CH3CH(CH3)COOH��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�