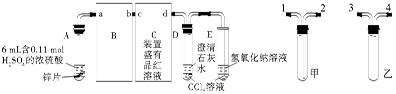��Ŀ����
N2�ڻ���������ũҵ��ҽ�ơ����캽�յ�������;�㷺�����ṩ����װ�ã�����������ȥ����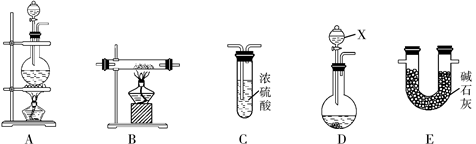
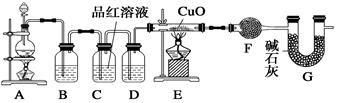
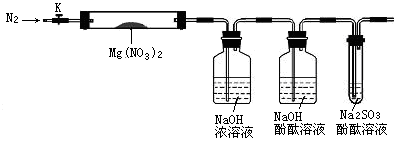
��ij��ѧ��ȤС���ͬѧ���ü���NaNO2��NH4Cl��Ũ��Һ�Ƶ�N2��Ӧ��ѡ��ķ���װ���� ��������ҩƷ����ȡ����ǰ������еIJ����� ��
���ڼ��������£���NH3��ԭCuO���Ƶ�N2��ͬʱ���ͭ�ۣ��˷�Ӧ�Ļ�ѧ��Ӧ����ʽ�� ��
����ͬѧ���â��з�Ӧԭ���Ʊ����������N2������Ҫ��NH3����ʯ�Һ�Ũ��ˮ��ԭ�ϡ������������ҵ�����˳����D��E��B��C������װ��D������X�������� ��װ��C���Լ��������� ��
��1��A ���������
��2��
��3����Һ©�� ���ﵪ������ȥ���еİ���
�������������ҺҺ��Ӧ��ѡ��Aװ�ã��̷̹�Ӧ��ѡ��Bװ�ã���Һ��Ӧ��ѡ��Dװ�á����ԣ�1��A ����ȡ����ǰ��Ҫ��һ���Ǽ��װ�õ������ԡ� ��2���������֪����������ͭ��Ӧ��������ͭ�͵�����ˮ��3��Xװ��Ϊ��Һ©����C��Ũ���������ˮ�Ժ����ԣ���˿����������ﵪ������ȥ���еİ���
���㣺���黯ѧʵ��Ļ������������֪ʶ��

 ��У����ϵ�д�
��У����ϵ�д���16�֣������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣬ֱ���ŷź�SO2���������γ����꣬Σ��������
��1���û�ѧ����ʽ��ʾSO2�γ�����������ķ�Ӧ�� ��2��
��2����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L-1��Na2SO3��Һ����ҺpH���ϼ�С������ҺpHԼΪ6ʱ������SO2�����������½���Ӧ�������ռ���
�� ��ʱ��Һ��c(SO32�C)��Ũ����0.2 mol��L-1������Һ��c(HSO3�C)��_______mol?L-1��
�� ��pHԼΪ6�����ռ���ͨ��������O2���ɽ����е�NaHSO3ת��Ϊ�������ʣ���Ӧ�Ļ�ѧ����ʽ�� ��2��
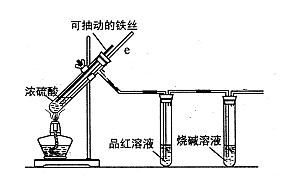
�� ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ��ģ��ʵ�����պ���������ʵ������ͼ��ʾ���� �����������SO2������Ч�ʡ�2��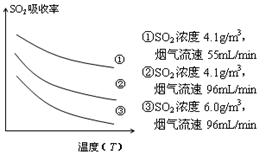
��3�������ֿ��ŵ�Na2SO3ҩƷ�Ѳ��ֱ������������û�ѧС��������֪Ũ�ȵ�����KMnO4��Һ��ȷ���京�������岽�����£�
����i����ȡ��Ʒ1.000 g��
����ii������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
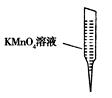
����iii����ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У���0.01000 mol��L��1 KMnO4����Һ�ζ����յ㡣
�����������������ظ�2�Ρ�
�� д������iii��������Ӧ�����ӷ���ʽ_________________________________��
�� ������0.01000 mol��L��1 KMnO4��Һʱ�����Ӷ��ݣ������ղ��ҩƷ��Na2SO3�ĺ���________(�ƫ����ƫС������Ӱ�족)��
�� ijͬѧ����������������еζ�ʵ��(�гֲ�����ȥ)�������������� (����ĸ)��

A B C D E
�� �ζ�������±���ʾ��
| �ζ����� | ������Һ �����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |

 ��
��
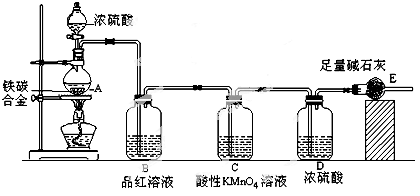
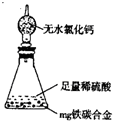
 ZnSO4��SO2����2H2O��ȡ22.4 L(��״��)SO2���塣ȡ65.0 gп����98%��ŨH2SO4(�ѣ�1.84 g��cm-3)110 mL��ַ�Ӧ��пȫ���ܽ⡣�����Ƶõ����壬��ͬѧ��Ϊ���ܻ���������Ϊ�ˣ���ѧС���ͬѧ���������ʵ��װ�ã�������ȡ���������̽����
ZnSO4��SO2����2H2O��ȡ22.4 L(��״��)SO2���塣ȡ65.0 gп����98%��ŨH2SO4(�ѣ�1.84 g��cm-3)110 mL��ַ�Ӧ��пȫ���ܽ⡣�����Ƶõ����壬��ͬѧ��Ϊ���ܻ���������Ϊ�ˣ���ѧС���ͬѧ���������ʵ��װ�ã�������ȡ���������̽����