��Ŀ����
��8�֣���100 mL��NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ���CO2�����V(��״��)��M������W�Ĺ�ϵ����ͼ��ʾ���Խ���������⣺
(1)A��ʱ����ɫ����M�Ļ�ѧʽΪ____________________��ͨ���CO2�����Ϊ________ mL (��״���£���ͬ)��
(2)C��ʱ����ɫ����M�Ļ�ѧʽΪ____________________��ͨ���CO2�����Ϊ________ mL��
(3)B��ʱM����ɳɷ�Ϊ________(�û�ѧʽ��ʾ)��ͨ���CO2�����Ϊ________ mL��
(4)��NaOH��Һ�����ʵ���Ũ��Ϊ________��
��1��Na2CO3��1120 ��2��NaHCO3�� 2240 ��3��Na2CO3��NaHCO3��1792 ��4��1.0mol/L
���������������ͼ֪NaOH����Ϊ4 g�����ʵ���Ϊ0.1 mol����ȫת��ΪNa2CO3ʱ��Na2CO3����Ϊ0.1mol�� ��106g/mol="5.3" g����ȫת��ΪNaHCO3ʱ��NaHCO3����Ϊ0.1mol��84g/mol="8.4" g����A���ɫ����MΪNa2CO3��C���ɫ����MΪNaHCO3����
��106g/mol="5.3" g����ȫת��ΪNaHCO3ʱ��NaHCO3����Ϊ0.1mol��84g/mol="8.4" g����A���ɫ����MΪNa2CO3��C���ɫ����MΪNaHCO3����
��1��������������֪��A���ɫ����MΪNa2CO3����CO2���Ϊ0.1 mol�� ��22.4L?mol-1="1.12L=1120" mL��
��22.4L?mol-1="1.12L=1120" mL��
��2��������������֪��C���ɫ����MΪNaHCO3����CO2���Ϊ0.1 mol��22.4L?mol-1="2.24L=2240" mL��
��3��ͼB��ʱM������Ϊ7.16 g��5.3��7.16��8.4��֪M��Na2CO3��NaHCO3��ɣ�����B��ʱNa2CO3���ʵ���Ϊx��NaHCO3���ʵ���Ϊy����2x+y��0.1��106x+84y��7.16�����x=0.02��y=0.06����V��CO2��=��0.02 mol+0.06 mol����22.4L?mol-1="1.792L=1792" mL��
��4����NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol��0.1L=1mol/L��
���㣺���������̼���������Ʒ�Ӧ���йؼ���

 һ����������ϵ�д�
һ����������ϵ�д��������ӷ���ʽ����ȷ����
| A��FeO����ϡ�����У�FeO+2H+ = Fe2++H2O |
| B������CO2ͨ��NaOH��Һ�У�OH¯+CO2 = HCO3¯ |
| C������������ˮ�У�Na+H2O = Na++OH¯+H2�� |
| D��������ˮ����AlCl3��Һ�У�Al3++3OH¯= Al(OH)3�� |
���£���Ӧ�����£����и�������һ���ܴ����������
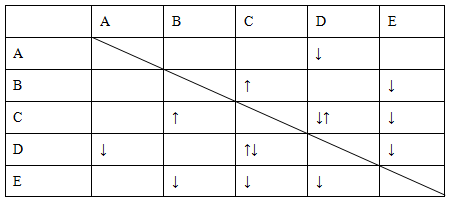
| A��c(Al3+)=0.1mol/L����Һ�У�Na+��K+��AlO2-��OH- |
| B����ɫ��Һ�У�K+��CH3COO-��HCO3-��MnO4- |
C��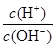 =1��1014����Һ��Ba2+��Na+��SO32-��NO3- =1��1014����Һ��Ba2+��Na+��SO32-��NO3- |
| D��ͨ������CO2���������ӻ��ܴ���������ǣ�K+��Ca2+��NO3-��Cl- |
�������ӷ���ʽ������ȷ����
| A������������ˮ�Ʊ������Cl2��H2O��2H����Cl����ClO�� |
| B����Fe(OH)2�м���������ϡHNO3��Fe(OH)2��2H����Fe2����2H2O |
| C����̼�������Һ�м�������������������Һ��NH4+��OH����NH3��H2O |
| D����������Һ�м������������������Һ��Al3����2SO42����2Ba2����4OH����2BaSO4����AlO2-��2H2O |
ijͬѧ��һ��ɫ����Һ���з����ó�����Һ�к�������ij�����ӣ�����Ϊ��������Ӧ���ǣ� ��
| A��Al3+��NO3�D��K+��SO42�� | B��Ca2+��H+��CO32����AlO2�D |
| C��OH����SO42����NH4+��Al3+ | D��Fe3+��Mg2+��NO3����Cl�� |
��15�֣�NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ����ҵ�ϳ�����������Ҫ�ɷ�ΪAl2O3�������� SiO2��Fe2O3���ʣ��������������
NH4Al(SO4)2?12H2O���乤������ͼ���£�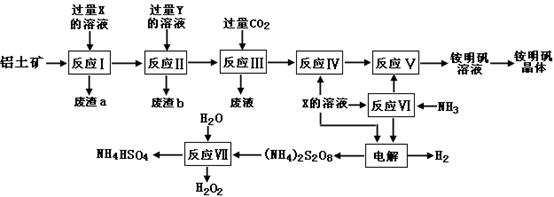
��1������a��b �ijɷֱַ��ǣ�_________��_____________����д���ƣ�
��2������ͼ��X�Ļ�ѧʽΪ��_______________��
��3����Ӧ������ӷ���ʽΪ��_________________________________________�����������Һ�л�����������IJ�������Ϊ����������ƣ�_________����ȴ�ᾧ������ϴ�ӡ�
��4���������[(NH4)2S2O8]�ڹ�ҵ�������й㷺����;��������Ϊ��������Ư�����㷺���������ع�ҵ���������ۺϵ�����������ά��ҵ���ѽ������������Ĺ����������ö��Ե缫���X�뷴Ӧ���������ʵĻ����Һ���Եõ�������李�
д��������Ӧʽ��________________________ ____��
��5����Ӧ���Ļ�ѧ����ʽΪ��_________________________ _____________��
NH4HSO4��Һ������Ũ���ɴ�С˳��Ϊ��__________________________ _��
��6�������������Һ����μ�������������Һ�������ܷ����ķ�Ӧ�� ����ѡ����ĸ��
| A��4NH4Al(SO4)2+3Ba(OH)2��2(NH4)2SO4+3BaSO4��+ Al2 (SO4)3+2Al(OH)3�� |
| B��2NH4Al(SO4)2+4Ba(OH)2��(NH4)2SO4+3BaSO4��+Ba(AlO2)2 |
| C��2NH4Al(SO4)2+3Ba(OH)2��(NH4)2SO4+3BaSO4��+2Al(OH)3�� |
| D��NH4Al(SO4)2+2Ba(OH)2��NH3��H2O+2BaSO4��+ Al(OH)3�� |
��10�֣����������ҹ���������̼���о����ش��չ���绡���ϳɵ�̼���ܣ������д������ʡ���̼������������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ:
C+ K2Cr2O7 + H2SO4(ϡ) CO2��+ Cr2 (SO4) 3+ + .
CO2��+ Cr2 (SO4) 3+ + .
��1���˷�Ӧ���������� ����������Ԫ���� ��
��2����ɲ���ƽ������Ӧ�Ļ�ѧ����ʽ��
��3��H2SO4��������Ӧ�б��ֳ����������� ����ѡ���ţ�
| A������ | B�������� | C����ˮ�� | D����ˮ�� |
��5��K2Cr2O7�����ڲⶨ�����εĺ���������FeSO4����0.4000�ˣ��ܽ��ữ����Ũ��Ϊ0.02000mol/L��K2Cr2O7����Һ�ζ������ı���Һ20.00mL�����������FeSO4����������Ϊ ��
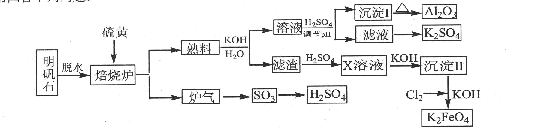
 2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .
2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .