��Ŀ����
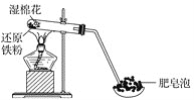
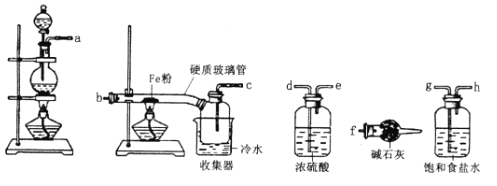
����Ŀ��ij��ѧ��ȤС��Ϊ����ľ̿��Ũ���ᷴӦ���ɵIJ�������ͼװ��(��֪SO2��ʹ��ˮ��ɫ)����ش�
(1)�Թ�D�з�����Ӧ�Ļ�ѧ����ʽΪ______________________��
(2)�Թ�B��������_________________________��
(3)F�Թ��г���ʯ��ˮ��������__________________________��
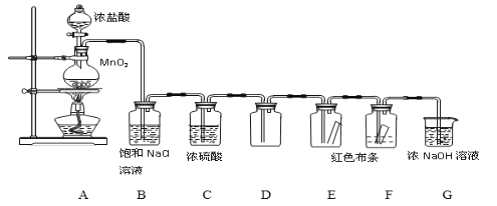
(��)ͬʱ��С���������ͼ��ʾ��ʵ��װ��(�̶�װ����)������SO2���ʵ��о�:
(4)��ͨ��SO2ʱ��A����ɫʯ����Һ��죬�û�ѧ����ʽ������ԭ����___________��
(5)��������֤SO2����Ư���Ե������ǣ�_______________________��
(6)��Ԫ�ػ��ϼ۵ĽǶȷ�����SO2�����ʣ�
�ٵ�ͨ��SO2һ��ʱ��ɹ۲�C����Һ��ɫ��˵��SO2����_____________�ԡ�
����Ҫ��֤SO2��������һ��������ʣ�Dװ���е�ҩƷӦΪ_____________��
A��FeCl3��ҺB����ˮC��������D��Ũ����
���𰸡�SO2��Br2��2H2O== H2SO4��2HBr ���� ���� �����д���CO2 SO2+H2O=H2SO3 B��Ʒ����Һ��ɫ ��ԭ C
��������
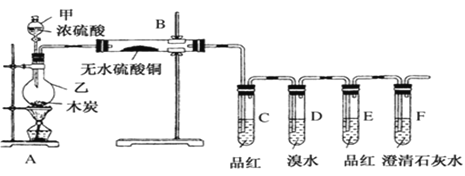
ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��C+2H2SO4![]() CO2��+2SO2��+2H2O����Ӧ������������CO2��SO2��H2O����ˮ����ͭ����ˮ�������Ϊ��ɫ�����������ɫ�����Ϊ��ɫ˵����ˮ���ɣ����Ʒ����ɫ˵����SO2���ɣ�Ҫȷ����CO2���ɣ���Ҫ����ʯ��ˮ����ǣ�����֮ǰ��Ҫ���ȳ�ȥSO2����Dװ������ˮ����SO2��Eװ����Ʒ����Һ����SO2�Ƿ���ȫ�����գ��ݴ˴��⡣
CO2��+2SO2��+2H2O����Ӧ������������CO2��SO2��H2O����ˮ����ͭ����ˮ�������Ϊ��ɫ�����������ɫ�����Ϊ��ɫ˵����ˮ���ɣ����Ʒ����ɫ˵����SO2���ɣ�Ҫȷ����CO2���ɣ���Ҫ����ʯ��ˮ����ǣ�����֮ǰ��Ҫ���ȳ�ȥSO2����Dװ������ˮ����SO2��Eװ����Ʒ����Һ����SO2�Ƿ���ȫ�����գ��ݴ˴��⡣
��1��Dװ������ˮ����SO2����Ӧ�����廯������ᣬ��ѧ����ʽΪSO2��Br2��2H2O=H2SO4��2HBr���ʴ�Ϊ��SO2��Br2��2H2O=H2SO4��2HBr��
��2����Ϊľ̿��Ũ���ᷴӦ��ˮ���ɣ���ˮ����ͭ����ˮ�������Ϊ��ɫ���ʴ�Ϊ��������
��3��F�Թ��г���ʯ��ˮ�������Ǽ�������д���CO2���ʴ�Ϊ����������д���CO2��
��4�����������ˮ��Ӧ���������ᣬ�������ܵ���������Ӷ�ʹ����Һ�����ԣ���ɫʯ����Һ������ɫ��������ɫʯ����Һ���ɫ���䷴Ӧ����ʽΪ��H2O+SO2=H2SO3���ʴ�Ϊ��SO2+H2O=H2SO3��
��5��SO2����Ư���ԣ���ʹƷ����Һ��ɫ��������֤SO2����Ư���Ե�������B��Ʒ����Һ��ɫ���ʴ�Ϊ��B��Ʒ����Һ��ɫ��
��6�������Ը�����ؾ���ǿ�����ԣ����������������������ᣬ�÷�Ӧ�ж�����������ԭ�������Զ���������л�ԭ�ԣ��ʴ�Ϊ����ԭ��
��Ҫ֤����������������ԣ������������ͻ�ԭ�����ʷ���������ԭ��Ӧ��
A.FeCl3��Һ���������ԣ���������������A����
B.��ˮ����ǿ�����ԣ���������������B����
C.��������л�ԭ�ԣ��ܱ�����������������C��ȷ��
D.Ũ�������ǿ�����ԣ���������������D����
�ʴ�Ϊ��C��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�����Ŀ����������ʵ��������������õ��Ľ�����ȷ����
ѡ�� | ʵ����������� | ʵ����� |
A | �����������������Ҵ���Һ���ȣ���������ͨ�����Ը��������Һ�У���ɫ | ����ϩ���� |
B | ���Թ��е�Ũ�������ͭƬ���Ǻý���������ͨ����Ʒ����Һ������������ | ͭƬδ��ĥ |
C | ��ȥCuSO4��Һ��Fe2+���ȼ�����H2O2���ټ�Cu(OH)2����ҺpH=4 | Ksp[Cu(OH)2]>Ksp[Fe(OH)2] |
D | ����ɫֽ������ʢ�����������ļ���ƿ�У����ϲ���Ƭ������������ | ����Ư�ײ���Cl2����ֱ�����õĽ�� |
A. A B. B C. C D. D