��Ŀ����
��1����4�֣����������У���CH2 =CHCH2OH����ͬϵ����� ����CH2 =CHCH2OH��Ϊͬ���칹����� ������ţ�
(2)��6�֣�ij�о�С�������������о���
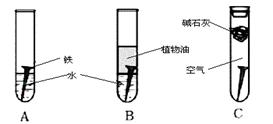
�����ϳ�ʱ���ͬѧ�۲쵽�������ǣ� ��ͼ�е������������������ ������ĸ�����������Ҫ�ɷ���
�ڸ���ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ
| A��CH3CH2OH | B��CH2 =CH��CH=CH��CH2��OH |
| C��CH3CH=CHCH2OH | D��CH3CH2CHO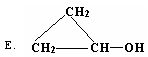 |

�����ϳ�ʱ���ͬѧ�۲쵽�������ǣ� ��ͼ�е������������������ ������ĸ�����������Ҫ�ɷ���
�ڸ���ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ
��1����4�֣�C DE
(2)��6�֣���A�� Fe2O3���� Fe2O3�� xH2O�� ��O2��2 H2O��4e���� 4 OH��
(2)��6�֣���A�� Fe2O3���� Fe2O3�� xH2O�� ��O2��2 H2O��4e���� 4 OH��
��1���ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ���л���ģ�����Ϊͬϵ�����C��ȷ������ʽ��ͬ�ṹ��ͬ�Ļ����ﻥΪͬ���칹�壬�����DE��ȷ��
��2����B��ֲ���Ϳ��Ը���������C�м�ʯ�ҿ������տ�����ˮ��CO2������������ʴ����A���������Ҫ�ɷ���Fe2O3���� Fe2O3�� xH2O��
��ԭ��ظ���ʧȥ���ӣ������õ����ӣ�������Һ�����Ժ��������Է�������������ʴ��������ӦʽΪO2��2 H2O��4e���� 4 OH����
��2����B��ֲ���Ϳ��Ը���������C�м�ʯ�ҿ������տ�����ˮ��CO2������������ʴ����A���������Ҫ�ɷ���Fe2O3���� Fe2O3�� xH2O��
��ԭ��ظ���ʧȥ���ӣ������õ����ӣ�������Һ�����Ժ��������Է�������������ʴ��������ӦʽΪO2��2 H2O��4e���� 4 OH����

��ϰ��ϵ�д�
�����Ŀ

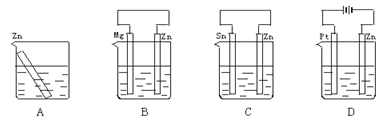
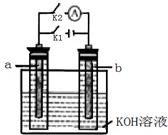
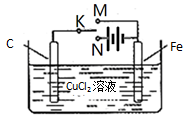
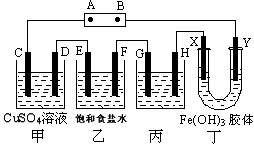
 �ܻ�ѧ����ʽ��
�ܻ�ѧ����ʽ�� _��
_��