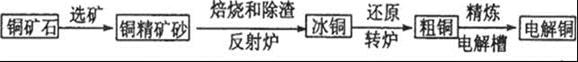
��Ŀ����
A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
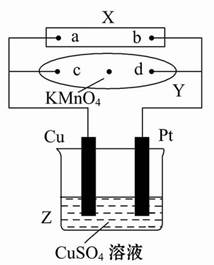
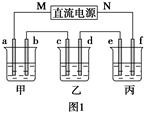
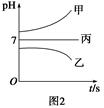
��ͼ1��ʾװ���У��ס��ҡ��������ձ�������ʢ��������A��Һ��������B��Һ��������C��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫����������16 g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵ��ͼ2��ʾ����ش��������⣺
 ��
��
��1��MΪֱ����Դ��________����b�缫�Ϸ����ĵ缫��ӦΪ________��
��2������e�缫�����ɵ������ڱ�״���µ����Ϊ________________��
��3��д�����ձ��е��ܷ�Ӧ�����ӷ���ʽ��_________________________________��
��4��Ҫʹ���ձ��е�C��Һ�ָ���ԭ����״̬����Ҫ���еIJ�����(д��Ҫ��������ʺ�����)______________________________��
| ������ | Na����K����Cu2�� |
| ������ | SO42-��OH�� |
��ͼ1��ʾװ���У��ס��ҡ��������ձ�������ʢ��������A��Һ��������B��Һ��������C��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫����������16 g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵ��ͼ2��ʾ����ش��������⣺
 ��
��
��1��MΪֱ����Դ��________����b�缫�Ϸ����ĵ缫��ӦΪ________��
��2������e�缫�����ɵ������ڱ�״���µ����Ϊ________________��
��3��д�����ձ��е��ܷ�Ӧ�����ӷ���ʽ��_________________________________��
��4��Ҫʹ���ձ��е�C��Һ�ָ���ԭ����״̬����Ҫ���еIJ�����(д��Ҫ��������ʺ�����)______________________________��
��1������4OH����4e��=2H2O��O2��
��2��5.6 L
��3��2Cu2����2H2O 2Cu��4H����O2��
2Cu��4H����O2��
��4��ˮ��4.5 g
��2��5.6 L
��3��2Cu2����2H2O
 2Cu��4H����O2��
2Cu��4H����O2����4��ˮ��4.5 g
�������⣬����ΪCuSO4��Һ��c�缫Ϊ������MΪ������NΪ��������ΪNa2SO4��K2SO4����ΪNaOH��KOH��Һ��bΪ�������缫��ӦʽΪ4OH����4e��=2H2O��O2����
��2��e�缫Ϊ�������缫��ӦʽΪ2H����2e��=H2�������ڱ�״���µ����Ϊ ��22.4 L��mol��1��5.6 L��
��22.4 L��mol��1��5.6 L��
��3��2Cu2����2H2O 2Cu��4H����O2����
2Cu��4H����O2����
��4�����Na2SO4��K2SO4��Һ��ʵ���ǵ��ˮ������Ӧ����ձ��м�ˮ��������Ϊ4.5 g��
��2��e�缫Ϊ�������缫��ӦʽΪ2H����2e��=H2�������ڱ�״���µ����Ϊ
 ��22.4 L��mol��1��5.6 L��
��22.4 L��mol��1��5.6 L����3��2Cu2����2H2O
 2Cu��4H����O2����
2Cu��4H����O2������4�����Na2SO4��K2SO4��Һ��ʵ���ǵ��ˮ������Ӧ����ձ��м�ˮ��������Ϊ4.5 g��

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
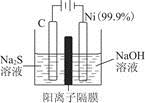
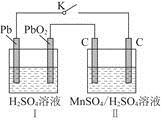
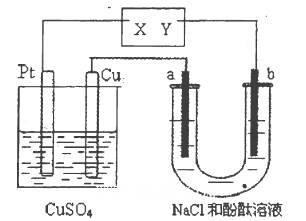
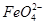 ��ʵ������У������������������Y������Һ������
��ʵ������У������������������Y������Һ������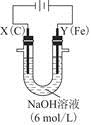
 KIO3 + 3H2��������˵������ȷ����
KIO3 + 3H2��������˵������ȷ����