��Ŀ����
�Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
��1������Ϊ���ı��洦����һ�ַ�����
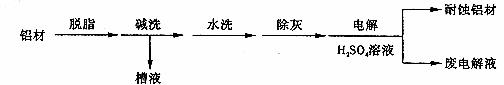
�ټ�ϴ��Ŀ����ϴȥ���ı������Ȼ����Ĥ����ϴʱ��������ð����ԭ�������� �������� �������ӷ���ʽ��ʾ����
Ϊ����ϴ��Һ�е����Գ�����ʽ���գ�������Һ�м��������Լ��е��������� ��
a��NH3�������� b��CO2 ����������c�� NaOH������ ����d��HNO3
��������Ϊ�������ڹ�����H2SO4��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦʽΪ������ ��ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ������ԭ���� ������������ �� �������������� �������ӷ���ʽ��ʾ����
��2����ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ����
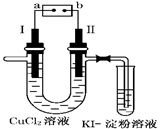
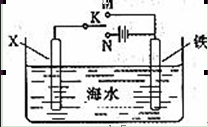
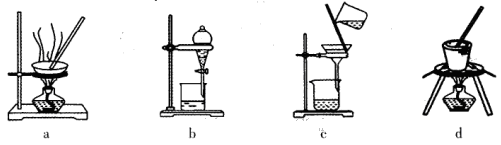
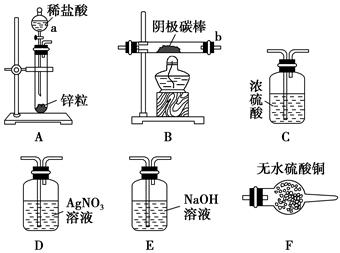
��3�����������װ�ã�����ģ�����ĵ绯ѧ��������XΪ̼����Ϊ�������ĸ�ʴ������KӦ�������� ������
��XΪп������K����M�����õ绯ѧ��������Ϊ������������������������������ ��
��1������Ϊ���ı��洦����һ�ַ�����

�ټ�ϴ��Ŀ����ϴȥ���ı������Ȼ����Ĥ����ϴʱ��������ð����ԭ�������� �������� �������ӷ���ʽ��ʾ����
Ϊ����ϴ��Һ�е����Գ�����ʽ���գ�������Һ�м��������Լ��е��������� ��
a��NH3�������� b��CO2 ����������c�� NaOH������ ����d��HNO3
��������Ϊ�������ڹ�����H2SO4��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦʽΪ������ ��ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ������ԭ���� ������������ �� �������������� �������ӷ���ʽ��ʾ����

��2����ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ����
��3�����������װ�ã�����ģ�����ĵ绯ѧ��������XΪ̼����Ϊ�������ĸ�ʴ������KӦ�������� ������
��XΪп������K����M�����õ绯ѧ��������Ϊ������������������������������ ��
��1����2Al+2OH��+2H2O��2AlO2��+3H2����1�֣� b(1��)
��2Al-6e��+3H2O��Al2O3��6H����2�֣�
HCO3���� H���� CO2��+ H2O ��2�֣�
Al3++3HCO3����Al(OH)3��+3CO2����2�֣�
��2��������Һ�����ĵ�Cu2+��������Һ��Cu2+Ũ�Ⱥ㶨����2�֣�
��3��N��1�֣���������������������������������������������1�֣�
��2Al-6e��+3H2O��Al2O3��6H����2�֣�
HCO3���� H���� CO2��+ H2O ��2�֣�
Al3++3HCO3����Al(OH)3��+3CO2����2�֣�
��2��������Һ�����ĵ�Cu2+��������Һ��Cu2+Ũ�Ⱥ㶨����2�֣�
��3��N��1�֣���������������������������������������������1�֣�
������������ļ��Կ���ϴȥ���ۣ�ͬʱҲ����ϴȥ���������������¶������ͼ���Һ��Ӧ�ų�������2Al+2OH��+2H2O��2AlO2��+3H2�������������������������������ǿ��ǿ���ˣ���Һ�е�ƫ�������Ӧͨ��CO2ʹ�����������������ѡb����������Ϊ�������ڹ�����H2SO4��Һ�е�⣬���ı����γ�����Ĥ����ʧ���ӷ���������Ӧ�������缫��ӦʽΪ2Al-6e��+3H2O��Al2O3��6H����ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ������ԭ����Һ�������ᣬ������������HCO3���� H���� CO2��+ H2O��Al3++3HCO3����Al(OH)3��+3CO2������2����ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ���Dz�����Һ�����ĵ�Cu2+��������Һ��Cu2+Ũ�Ⱥ㶨����3����XΪ̼����Ϊ�������ĸ�ʴ��Ӧ���ɵ��أ������ĵ籣����������KӦ������N������XΪп������K����M�����õ绯ѧ��������Ϊ����������������������

��ϰ��ϵ�д�
�����Ŀ
 ��
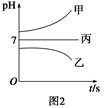
��

 Cr2O3)ұ�����Ĺ�����������:
Cr2O3)ұ�����Ĺ�����������: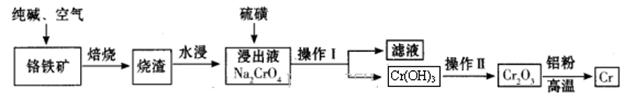
 Cr2O72����H2O����
Cr2O72����H2O����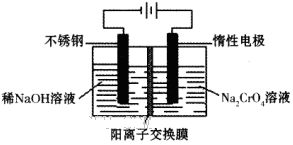


 O2��+2H2O
O2��+2H2O