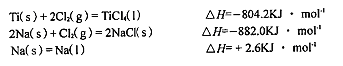��Ŀ����
��1��һ������﮵���ǽ���ѧʽΪLi4Ti5O12��������Ϊ��ص��������ϣ��ڷŵ�Ĺ����б�Ϊ��ѧʽΪLi4Ti5O12�����ʣ�
��Li4Ti5O12��TiԪ�صĻ��ϼ�Ϊ ��﮵�ص�ͻ���ŵ��� ��
�ڸ�﮵����һ�ֶ��ε�أ��ŵ�ʱ�ĸ�����ӦʽΪ �����ʱ��������ӦʽΪ ��
��2����������ԭ�ζ����ⶨ�Ʊ��õ���TiO2�����е�TiO2��������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+������KSCN��Һ��Ϊָʾ������NH4Fe��SO4��2����Һ�ζ�Ti3+��ȫ������Ti4+��
��TiCl4ˮ������TiO2?xH2O�Ļ�ѧ����ʽΪ ��
�ڵζ��յ�������� ��
�۵ζ�����ʱ����ȡTiO2����0.2g������0.1mol?L-1 NH4Fe��SO4��2����Һ20ml����TiO2����������Ϊ ��
�����ڵζ��յ㣬��ȡ�ζ��̶ܿ�ʱ�����ӱ���Һ��Һ�棬ʹ��ⶨ��� ���ƫ����ƫС������Ӱ�족��o
��3����֪��
Ti��s��+2Cl2��g��=TiCl4��l����H=-804.2kJ?mol-1
2Na��s��+Cl2��g��=2NaCl��s����H=-882.0kJ?mol-1
Na��s��=Na��l����H=+2.6kJ?mol-1
��TiCl4��l��+4Na��l��=Ti��s��+4NaCl��s���ġ�H= KJ?mol-1��
��Li4Ti5O12��TiԪ�صĻ��ϼ�Ϊ
�ڸ�﮵����һ�ֶ��ε�أ��ŵ�ʱ�ĸ�����ӦʽΪ
��2����������ԭ�ζ����ⶨ�Ʊ��õ���TiO2�����е�TiO2��������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+������KSCN��Һ��Ϊָʾ������NH4Fe��SO4��2����Һ�ζ�Ti3+��ȫ������Ti4+��
��TiCl4ˮ������TiO2?xH2O�Ļ�ѧ����ʽΪ
�ڵζ��յ��������
�۵ζ�����ʱ����ȡTiO2����0.2g������0.1mol?L-1 NH4Fe��SO4��2����Һ20ml����TiO2����������Ϊ
�����ڵζ��յ㣬��ȡ�ζ��̶ܿ�ʱ�����ӱ���Һ��Һ�棬ʹ��ⶨ���
��3����֪��
Ti��s��+2Cl2��g��=TiCl4��l����H=-804.2kJ?mol-1
2Na��s��+Cl2��g��=2NaCl��s����H=-882.0kJ?mol-1
Na��s��=Na��l����H=+2.6kJ?mol-1
��TiCl4��l��+4Na��l��=Ti��s��+4NaCl��s���ġ�H=
��������1���ٸ��ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0ȷ��TiԪ�صĻ��ϼۣ��Ԫ�ص����ԭ��������С��
�ڷŵ�ʱ���������ʧ���ӷ���������Ӧ�����ʱ��������ʧ���ӷ���������Ӧ��
��2����TiCl4��ǿ�������Σ�ˮ������TiO2?xH2O����Ӧ���
�������������軯����Һ���Ѫ��ɫ��
�۸���ת�Ƶ�������ȼ���������ѵ��������ٸ�������������ʽ���м��㼴��
�ܸ���Һ�棬���¶���ƫС��
��3�����ݸ�˹���ɽ��м��㣮
�ڷŵ�ʱ���������ʧ���ӷ���������Ӧ�����ʱ��������ʧ���ӷ���������Ӧ��
��2����TiCl4��ǿ�������Σ�ˮ������TiO2?xH2O����Ӧ���
�������������軯����Һ���Ѫ��ɫ��
�۸���ת�Ƶ�������ȼ���������ѵ��������ٸ�������������ʽ���м��㼴��
�ܸ���Һ�棬���¶���ƫС��
��3�����ݸ�˹���ɽ��м��㣮
����⣺��1���ٸû������У�OԪ�صĻ��ϼ�Ϊ-2�ۣ��Ԫ�صĻ��ϼ�Ϊ+1�ۣ����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0֪��TiԪ�صĻ��ϼ�=2��12-1��4=
=+4���Ԫ�ص����ԭ��������С��������ͬ������﮵�أ������С�������ߣ�Я�����㣬�ʴ�Ϊ��+4�����С���������ߡ�Я�����㣻
�ڷŵ�ʱ���������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Li-e-=Li+�����ʱ��������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Li7Ti5O12-3e-=Li4Ti5O12+3Li+��
�ʴ�Ϊ��Li-e-=Li+��Li7Ti5O12-3e-=Li4Ti5O12+3Li+��
��2����TiCl4��ǿ�������Σ�ˮ������TiO2?xH2O���Ȼ��⣬ˮ�ⷴӦ����ʽΪ��TiCl4+��x+2��H2O=TiO2��xH2O+4HCl��
�ʴ�Ϊ��TiCl4+��x+2��H2O=TiO2��xH2O+4HCl��
�������������軯����Һ���Ѫ��ɫ����������ӱ���ԭ�����������ӣ�����Һ������ɫ��Ϊ��ɫ���ʴ�Ϊ����Һ��ɺ�ɫ��
�۸���������ԭ��Ӧ�е�ʧ������ȵö��ߵĹ�ϵʽ��TiO2----NH4Fe��SO4��2���������ѵ�����=
��80g/mol=0.16g������������=
��100%=80%��
�ʴ�Ϊ��80%��
�ܸ���Һ�棬���¶���ƫС��ʹ��ⶨ���ƫС���ʴ�Ϊ��ƫС��
��3�����ݸ�˹���ɵ�
Ti��s��+2Cl2��g��=TiO4��l����H=-804.2kJ?mol-1��
2Na��s��+Cl2��g��=2NaCl��s����H=-882.0kJ?mol-1 ��
Na��s��=Na��l����H=+2.6kJ?mol-1 ��
������ʽ2��-��-4�۵�TiCl4��l��+4Na��l��=Ti��s��+4NaCl��s������H=2��-882.0kJ?mol-1��-4��+2.6kJ?mol-1��-��-804.2kJ?mol-1��=-970.2kJ?mol-1��
�ʴ�Ϊ��-970.2��
| 2��12-1��4 |
| 5 |
�ڷŵ�ʱ���������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Li-e-=Li+�����ʱ��������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Li7Ti5O12-3e-=Li4Ti5O12+3Li+��
�ʴ�Ϊ��Li-e-=Li+��Li7Ti5O12-3e-=Li4Ti5O12+3Li+��
��2����TiCl4��ǿ�������Σ�ˮ������TiO2?xH2O���Ȼ��⣬ˮ�ⷴӦ����ʽΪ��TiCl4+��x+2��H2O=TiO2��xH2O+4HCl��
�ʴ�Ϊ��TiCl4+��x+2��H2O=TiO2��xH2O+4HCl��
�������������軯����Һ���Ѫ��ɫ����������ӱ���ԭ�����������ӣ�����Һ������ɫ��Ϊ��ɫ���ʴ�Ϊ����Һ��ɺ�ɫ��
�۸���������ԭ��Ӧ�е�ʧ������ȵö��ߵĹ�ϵʽ��TiO2----NH4Fe��SO4��2���������ѵ�����=
| 0.1mol/L��0.02L��1 |
| 1 |
| 0.16g |
| 0.2g |
�ʴ�Ϊ��80%��
�ܸ���Һ�棬���¶���ƫС��ʹ��ⶨ���ƫС���ʴ�Ϊ��ƫС��
��3�����ݸ�˹���ɵ�
Ti��s��+2Cl2��g��=TiO4��l����H=-804.2kJ?mol-1��
2Na��s��+Cl2��g��=2NaCl��s����H=-882.0kJ?mol-1 ��
Na��s��=Na��l����H=+2.6kJ?mol-1 ��
������ʽ2��-��-4�۵�TiCl4��l��+4Na��l��=Ti��s��+4NaCl��s������H=2��-882.0kJ?mol-1��-4��+2.6kJ?mol-1��-��-804.2kJ?mol-1��=-970.2kJ?mol-1��
�ʴ�Ϊ��-970.2��
�����������漰ԭ���ԭ�������ӵļ��顢��˹���ɵ�֪ʶ�㣬��ȷ��˹���ɵĺ��塢ԭ��غ͵���ԭ�����ɽ��ע��ζ��ܶ�������Ͳ����������Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
 ���⡱������Ҫ�Ļ���ԭ�ϣ�Ҳ��δ�������������Դ��
���⡱������Ҫ�Ļ���ԭ�ϣ�Ҳ��δ�������������Դ��