��Ŀ����
����Ŀ����1���ҹ�����������Դ����������Դ�ĸ�Чת����ѭ�����á����������������
A�����շϾɵ�أ�����ҪĿ���ǻ������еĽ���
B��������չú��������Һ������
C���þ���ϩ���ϴ�����������Ͽɼ��ٰ�ɫ��Ⱦ
D���ӿ�ʯ�͵Ȼ�ʯȼ�ϵĿ��ɺ�ʹ��
E����CO2Ϊԭ�������ɽ�������
F�����ոѽ��мӹ�ת��Ϊ�Ҵ�ȼ��
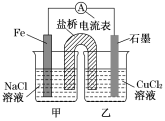
��2���ö��Ե缫�ֱ������и����ʵ�ˮ��Һһ��ʱ�����ʣ����Һ�м�������ˮ��ʹ��Һ�ָ������ǰŨ�ȵ���
A��NaCl B��H2SO4 C��CuCl2 D��AgNO3
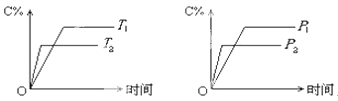
��3��Mg-H2O2��ؿ������������˼�ʻ��DZ�������õ���Ժ�ˮΪ�������Һ��ʾ��ͼ���¡��õ�ع���ʱ��ʯī�缫������Һ��pH ��������С�����䣩

��4��һ�������£����ȼ��һ�����Ķ���ų�����ΪQ kJ�����ⶨ��ȫ����ȼ�պ����ɵĶ�����̼����������5 mol��L��1��KOH��Һ100 mL����ǡ���������Ρ���������·�Ӧ��C4H10(g)��![]() O2(g)=4CO2(g)��5H2O(l)�Ħ�H ��
O2(g)=4CO2(g)��5H2O(l)�Ħ�H ��
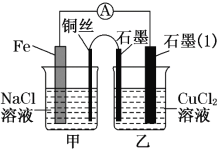
��5���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ�����ϵ��¹��տ��Խ���30%���ϡ������ֹ�������У�������ϵĴ�����ת����ϵ��ͼ��ʾ�����мס��ҡ���������Ϊʯī�缫��

����װ����ͨ��0.5 mol e�����������ϼ缫����X��������Ϊ__________L����״���£�
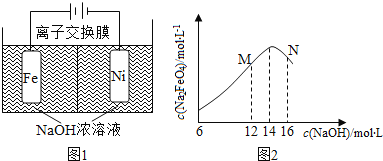
��ͼ��NaOH��Һ����������a����b����c���ɴ�С��˳��Ϊ________________________
���𰸡���1��ACD ��2��B ��3������ ��H����16QkJ/mol
��5���� 5.6L����״���£� ��b%�� a% ��c%
��������
�����������1��A�����շϾɵ�أ�����ҪĿ������ֹ�ؽ�����Ⱦ��A������B����߽ྻúȼ�ռ�����úҺ�����������Խ�����Ⱦ����ŷţ������ڻ���������B��ȷ��C���þ���ϩ���ϴ�������������������ٰ�ɫ��Ⱦ��C����D�����û�ʯȼ��ȼ�ջ������Ⱦ�������ӿ컯ʯȼ�ϵĿ��ɺ�ʹ�ã���ʯȼ��ȼ�ջ������Ⱦ�������ڻ�����������������ɫ��ѧ���D������E���ѽ������ϵ�ʹ���������ɫ��Ⱦ����CO2 Ϊԭ�������ɽ������ϣ��ܼ��ٰ�ɫ��Ⱦ�������ڻ���������E��ȷ��F�������ոѽ��мӹ�ת��Ϊ�Ҵ�ȼ�ϣ����Ϊ�������Ͽɳ�����չ������F��ȷ����ѡACD��
��2��A������Ȼ���ʵ���ǵ缫ˮ���Ȼ��ƣ�����������ʣ����Һ�м�����ˮ������ʹ��ҺŨ�Ⱥ͵��ǰ��ͬ��A����B�������������Һʵ���ǵ缫ˮ������������ʣ����Һ�м�����ˮ����ʹ��ҺŨ�Ⱥ͵��ǰ��ͬ��B��ȷ��C������Ȼ�ͭʵ���ǵ���Ȼ�ͭ������������ʣ����Һ�м�����ˮ������ʹ��ҺŨ�Ⱥ͵��ǰ��ͬ��C����D�����Ե缫�����������Һ��������������ˮ�����������ˮ���ܻظ�ԭ��Һ��Ũ�ȣ�D����ѡB��
��3���õ�ع���ʱþʧȥ���ӣ���������ʯī�缫��������˫��ˮ�õ����ӣ��缫��ӦʽΪH2O2��2e����2OH��������ʯī�缫������Һ��pH������
��4���������������ʵ�����0.5mol������̼�����0.25mol����˵����Ӧ������CO2��0.25mol���������4molCO2�ų���������16QkJ�����������·�ӦC4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l)����H����16QkJ/mol��
��5�����缫X�������ӷŵ������������װ����ͨ��0.5 mol e�����������ϼ缫����X��������Ϊ0.25mol��22.4L/mol��5.6L��
����ȼ�ϵ���У�����������������ͨ������ļ���������ͨ��ȼ�ϵļ��Ǹ�����������Y�ļ���������������������������X��������������������ȼ�ϵ������������ˮ������a%С��b%�������ڲ���������ͬʱ���������������ɣ�����c%��a%������С˳��Ϊb%��a%��c%��
ͼ��NaOH��Һ����������a����b����c���ɴ�С��˳��Ϊ

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�