��Ŀ����
�����ʽṹ�����ʡ�
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����2�֣�CH3������CH3��CH3��������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����
A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǵĿռ乹����ͬ��̼ԭ�Ӿ����Բ�ȡsp2�ӻ�
C��CH3����NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3���е�̼ԭ�Ӳ�ȡsp3�ӻ�������ԭ�Ӿ�����
��2����3�֣��ڼ��Է����У����������ͬ��������ļ�ľ����ż������ͨ����d��ʾ�����Է��ӵļ���ǿ��ͬż������������������ĵĵ�����q���йأ�һ����ż���أ��̣������������ӵ�ż���ض���Ϊż������ż����һ�˵�ɵ����ij˻������̣�d��q���Իش��������⣺
��HCl��CS2��H2S��SO2���ַ����Ц̣�0���� ��
��ʵ���ã���PF3��1.03����BCl3��0���ɴ˿�֪��PF3������ ���ͣ�BCl3������ ���͡�
��3����2�֣������ʵĴ����о����������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���__________________��
��4����3�֣����ù��������֤ʵ���ڽྻ���������ںϳɰ�
��Ӧ�Ĵ������ı����ϴ��ڵ�ԭ�ӣ���ͼΪ��ԭ�������ľ����ϵĵ��㸽�žֲ�ʾ��ͼ��ͼ��С��ɫ�������ԭ�ӣ���ɫ�������ԭ�ӣ������ڵ��㾧����N/Feԭ����֮��Ϊ________________��

��5����2�֣���������Ľṹ���õȾ�Բ����ܶѻ����������ڵȾ�Բ������ܶѻ��ĸ�����ʽ�У��������ܶѻ����������ܶѻ���Ϊ��Ҫ����ָ����ͼ���ĸ�Ϊ�������ܶѻ� ���A����B����

ͼA ͼB
��1��C��2�֣�
��2����CS2 ��1�֣� �������Σ�1�֣� ƽ�������Σ�1�֣�
��3��CrO2��2�֣�
��4��1��2��3�֣���5��A��2�֣�
��������



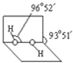 ��4����֪����������ӵĿռ�ṹ��ͼ��ʾ����������ԭ�Ӳ�ȡ
��4����֪����������ӵĿռ�ṹ��ͼ��ʾ����������ԭ�Ӳ�ȡ