��Ŀ����
����Ŀ��̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1MgSO4��Һ��0.5mol��L-1NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL�Ŀ���ƿ�У��������������¶ȿ�����50����
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40������ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1~5����
������2�����¶���50�����Ϻõļ��ȷ�����__________��
������3����MgCO3��nH2O���������ӷ���ʽΪ________��
������4�����Ƿ�ϴ�Ӹɾ��ķ�����___________��
��2���ⶨ�ϳɵ�MgCO3��nH2O�е�nֵ��
����1.000g̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м���ˮ����ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4~5h����Ӧ���ڽ��¶�����30���������ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�
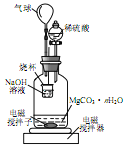
��ͼ�������������___________��
��������Ӧ����Ҫ���µ�30������ҪĿ����_________��
����3��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol����nֵΪ______���ú�a�ı���ʽ��ʾ����
��3����ȡ100g���������Ʒ�������ط���������������ͼ��

��������ºϳɵľ����У�n=______��ѡ�1��2��3��4��5����
���𰸡�ˮԡ���� Mg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+ ȡ���һ��ϴ��Һ���������������ữ��BaCl2��Һ���������ֳ�������ϴ�Ӹɾ� ��ʱ����CO2��������CO2��NaOH��Һ���գ����ܱ���װ����ѹǿ����ȶ� �����¶�������ܽ�ȼ�С��ʹ�ܽ���ˮ�е�CO2�ݳ�������������ȫ ��1.000-84a��/18a 1
��������
��1���٢�ˮԡ�������Ⱦ��ȣ����ڿ��ƣ�������100����ɲ���ˮԡ���ȣ�
�ʴ�Ϊ��ˮԡ���ȣ�
�ڽ�250mL MgSO4��Һ��μ���NH4HCO3��Һ�У��ð�ˮ������ҺpH��9.5����Ӧ����̼��þ�ᾧˮ�����Ӧ�Ļ�ѧ����ʽΪMg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+��
�ʴ�Ϊ��Mg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+��
��2����װ����������Ի���ѹǿ�����װ�ã���ʱ����CO2��������CO2��NaOH��Һ���գ����ܱ���װ����ѹǿ����ȶ���
�ʴ�Ϊ����ʱ����CO2��������CO2��NaOH��Һ���գ����ܱ���װ����ѹǿ����ȶ����ڷ�Ӧ���ڽ��¶�����30�棬ʹ���ɵĶ�����̼ȫ���ݳ�������������Һ���գ����ٲⶨ��������
�ʴ�Ϊ�������¶�������ܽ�ȼ�С��ʹ�ܽ���ˮ�е�CO2�ݳ�������������ȫ��
��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol������Ԫ���غ��֪��̼��þ���ʵ���Ϊamol�����ݻ�ѧʽ��֪��MgCO3nH2O��̼��þ������̼��þ�ͽᾧˮ���ʵ���֮��Ϊ1��n���õ�1��n=a��![]() ���õ�n=��1.000-84a��/18a���ʴ�Ϊ����1.000-84a��/18a��
���õ�n=��1.000-84a��/18a���ʴ�Ϊ����1.000-84a��/18a��
��3��MgCO3nH2O��n=1��5������ͼ��400��Cʣ������Ϊ82.3g��Ϊʧȥ�ᾧˮ��������ʣ������Ϊ39.5g��̼��þ�ֽ�ʧȥ������̼���������õ�100g��![]() =100-82.3�����n=1��
=100-82.3�����n=1��
�ʴ�Ϊ��1��

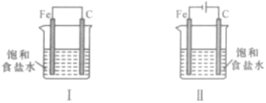
 ������������ϵ�д�
������������ϵ�д�����Ŀ����O2��HClת��ΪCl2�������Ч�棬������Ⱦ��
��1����ͳ�ϸ�ת��ͨ����ͼ��ʾ�Ĵ���ѭ��ʵ�֣�
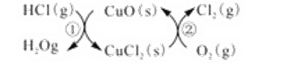
���У���Ӧ��Ϊ��2HCl��g�� + CuO��s��![]() H2O��g��+CuCl2��g�� ��H1
H2O��g��+CuCl2��g�� ��H1
��Ӧ������1molCl2��g���ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ___________����Ӧ������H1����H2��ʾ����
��2������RuO2����������HClת��ΪCl2���ܷ�Ӧ���и��õĴ����ԣ�
��ʵ������һ��ѹǿ�£��ܷ�Ӧ��HClƽ��ת�������¶ȱ仯��aHCl��T������ͼ�����ܷ�Ӧ����H___0������������������������������A��B�����ƽ�ⳣ��K��A����K��B���нϴ����_______��
��������ʵ������ѹ�����ʹѹǿ��������ӦaHCl��T���ߵ�ʾ��ͼ������Ҫ˵������______________��
�����д�ʩ�����������aHCl����___________��
A������n��HCl�� B������n��O2��
C��ʹ�ø��õĴ��� D����ȥH2O
��3��һ�������²�÷�Ӧ������n��Cl2�����������£�
t��min�� | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n��Cl2��/10-3mol | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
����2.0��6.0min����HCl�����ʵ����仯��ʾ�ķ�Ӧ����______����mol��min-1Ϊ��λ��д��������̣���
��4��Cl2��;�㷺��д����Cl2�Ʊ�Ư�۵Ļ�ѧ����ʽ______________��