题目内容
如图为电解装置,X、Y为电极材料,a为电解质溶液。
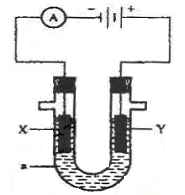
(1)若a为含有酚酞的KCl溶液,X为Fe,Y为石墨,电解一段时间后:
X电极附近可观察到的实验现象是 ;
写出Y电极的电极反应式 。
(2)若要实现Cu +H2SO4=CuSO4+H2↑,
则Y电极材料是 ;
写出X电极的电极反应式 。
(3)若要利用该装置在铁制品表面镀上一层银,则a为 ,反应前两电极的质量相等,反应后电极质量相差2.16g,则该过程理论上通过电流表的电子数为 。
(4)若X、Y均为惰性电极,a为NaOH溶液,电解一段时间后,溶液的pH (填“增大”“不变”“减小”),若要使溶液恢复原来的状态,可往溶液中加入 。

(1)若a为含有酚酞的KCl溶液,X为Fe,Y为石墨,电解一段时间后:
X电极附近可观察到的实验现象是 ;
写出Y电极的电极反应式 。
(2)若要实现Cu +H2SO4=CuSO4+H2↑,
则Y电极材料是 ;
写出X电极的电极反应式 。
(3)若要利用该装置在铁制品表面镀上一层银,则a为 ,反应前两电极的质量相等,反应后电极质量相差2.16g,则该过程理论上通过电流表的电子数为 。
(4)若X、Y均为惰性电极,a为NaOH溶液,电解一段时间后,溶液的pH (填“增大”“不变”“减小”),若要使溶液恢复原来的状态,可往溶液中加入 。
(1)电极表面产生气体,附近溶液变红 2分 2Cl- -2e-=Cl2↑ 2分
(2)Cu (1分) 2H++2e-=H2↑ 2分
(3)AgNO3溶液 (1分) 0.01NA或6.02×1021 2分
(4)增大 H2O 各2分
(2)Cu (1分) 2H++2e-=H2↑ 2分
(3)AgNO3溶液 (1分) 0.01NA或6.02×1021 2分
(4)增大 H2O 各2分
试题分析:根据装置可知,该装置是电解池,Y电极与电源的正极相连,做阳极,X电极与电源的负极相连,做阴极。
(1)若a为含有酚酞的KCl溶液,X为Fe,Y为石墨,则阳极Y是溶液中的氯离子放电生成氯气,电极反应式为2Cl- -2e-=Cl2↑;X电极是溶液中的氢离子放电生成氢气,由于氢离子放电破坏了阴极周围水的电离平衡,所以阴极周围溶液中OH-浓度大于氢离子浓度,溶液显碱性,则X电极附近可观察到的实验现象是电极表面产生气体,附近溶液变红。
(2)根据反应式Cu +H2SO4=CuSO4+H2↑可知,铜失去电子,溶液中的氢离子得到电子,所以若要实现Cu +H2SO4=CuSO4+H2↑,则铜做阳极,因此Y电极材料是铜,X电极是阴极,溶液中的氢离子放电,电极反应式为2H++2e-=H2↑。
(3)电镀时待镀金属做阴极,镀层金属做阳极,含有镀层金属离子的溶液做电镀液,所以若要利用该装置在铁制品表面镀上一层银,则a为硝酸银溶液。反应前两电极的质量相等,反应后电极质量相差2.16g,这说明阳极银有1.08g失去电子,而阴极有1.08g银析出,所以该过程理论上通过电流表的电子数为
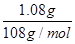 ×6.02×1023/mol=6.02×1021。
×6.02×1023/mol=6.02×1021。(4)若X、Y均为惰性电极,a为NaOH溶液,则阳极是溶液中的OH-放电,阴极是氢离子放电,这说明相当于是电解水,所以电解一段时间后,氢氧化钠的浓度增大,溶液的pH增大。由于相当于电解水,所以若要使溶液恢复原来的状态,可往溶液中加入水。

练习册系列答案
 世纪百通期末金卷系列答案
世纪百通期末金卷系列答案
相关题目
 Cu+Cl2↑
Cu+Cl2↑