��Ŀ����
����Ŀ��������(Ga)λ�����ڱ��ĵ������ڣ���Alͬ���壬��Ҫ����Ga3+��GaO2-����������ʽ�����㷺Ӧ���ڵ��ӹ�ҵ��
��1�������ۺ�Fe2O3�����ȷ�Ӧʵ�飬��Ҫ���Լ�����__________________��
a��KClO3 b. KCl c. MnO2 d. Mg
��2���뵼����ϵ�������Ga��NH3��һ�������·����û���Ӧ���ɡ��ù���ÿ����1molGaN(s)�ų�����15.4kJ���������ڱ��е����_____________��д���÷�Ӧ���Ȼ�ѧ����ʽ_________________��
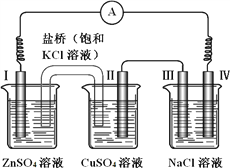
��3����ҵ���õ�ⷨ�����ء�����ԭ����ͼ��ʾ����֪�������Ļ��Zn>Ga>Fe>Cu

�ٵ�⾫����һ��ʱ����γɵ���������Ҫ��______________
����֪����ʱ������Ӧ��GaO2-+3e-+2H2O=Ga+4OH- �������ĵ缫��Ӧ����ʽ��___________________________________��
�����÷���Ƥ��ȡ����(Fe2O3)�IJ�������ʾ��ͼ���£�

��4������I�¶Ȳ��ܹ��ߡ���Ϊ��_______________________________��
��5������II�з�����Ӧ4Fe(NO3)2��O2��(2n��4)H2O��2Fe2O3��nH2O��8HNO3�����ɵ������ֽ�����Ƥ�е���ת��ΪFe(NO3)2��
д�����߷�Ӧ�����ӷ���ʽ______________________________________��
��6���������������У�����������ɫ��ѧ��˼�����______________________ ��
���𰸡� ad 31 2Ga(s)+ 2NH3(g) ��2GaN(s)+3H2(g) ��H��-30.8kJ/mol Fe��Cu Ga-3e-+4OH-=GaO2-+2H2O �¶ȹ��������ֽ� 4Fe��10H+ + NO3-��4 Fe2+��NH4+��3H2O ���������ŷ���
����������1�������ȷ�Ӧʵ��ʱ��ʹ��Mg��KClO3����ȼ����ad��ȷ����ȷѡ��ad��
��2����λ��Ԫ�����ڱ��е������ڣ�����ͬ���壬ԭ������Ϊ13+18=31��Ga��NH3��һ�������·����û���Ӧ���ɵ����غ�����������1molGaN(s)�ų�����15.4kJ��������Ӧ���Ȼ�ѧ����ʽ��2Ga(s)+ 2NH3(g) ��2GaN(s)+3H2(g) ��H�� -30.8kJ/mol����ȷ�𰸣�31��2Ga(s)+ 2NH3(g) ��2GaN(s)+3H2(g) ��H�� -30.8kJ/mol��
��3���ٸ��ݽ����Ļ��Zn>Ga>Fe>Cu��п��ʧ���ӱ�Ϊ������Ȼ��Gaʧ��������Ϊ��������Ga��Ӧ��ȫ����ʣ�������ͭ�����������ײ����γ�����������ȷ����Fe��Cu��
��������������������ʧ�������ڼ��Ի���������GaO2-���缫��Ӧ����ʽ��Ga-3e-+4OH-=GaO2-+2H2O����ȷ����Ga-3e-+4OH-=GaO2-+2H2O��
����4��������в��ȶ��ԣ����Ⱥ������ֽ⣬��˲���I�¶Ȳ��ܹ�������ȷ�����¶ȹ��������ֽ���
��5���������̿�֪������ϡ���ᷴӦ������������������泥���Ӧ�����ӷ���ʽΪ��4Fe��10H++NO3-��4 Fe2+��NH4+��3H2O����ȷ����4Fe��10H+ + NO3-��4 Fe2+��NH4+��3H2O��
��6�������е�Ԫ�صĻ�ԭ����Ϊ����������������ŷ������Ի�����Ⱦ��С����ȷ�������������ŷ�����

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�