��Ŀ����
A��B��C��D��Ϊ��ѧ��ѧ�г����ĵ��ʳɻ��������֮��Ĺ�ϵ��ͼ1��ʾ�����ֲ�������ȥ����
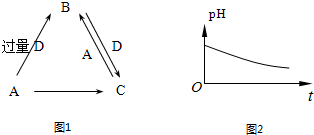
��1����AΪǿ�DΪ��̬�������Ϊ�����еijɷ֣�д��D�ĵ���ʽ______����ӦA+D����������C�����ӷ���ʽΪ��
______
��2����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ����о�ˮ���ã���
��A�еĽ���Ԫ�������ڱ���λ��______���ڡ���______�壮
�ڷ�ӦC+D��B�����ӷ���ʽΪ______��
��3����AΪ�������ʣ�D��ijǿ���ϡ��Һ����ӦC+D��B�����ӷ���ʽΪ______��
��4����AΪǿ�DΪ��̬�������Ϊ������Ⱦ��֮һ������ʱ����B��ˮ��Һ¶���ڿ����У���pH��ʱ��t�仯��ͼ2��ʾ������D���ܽ��ˮ�Ļӷ���������pH�仯��ԭ����______�������ӷ���ʽ��ʾ������֪��ͼ��y��7��B����ɫ��ӦΪ��ɫ����B��Һ�и�����Ũ���ɴ�С��˳����______��
�⣺��1����AΪǿ�DΪ��̬�������Ϊ�����еijɷ֣��ͼӦ˵����CO2��������̼�ĵ���ʽΪ ����ӦA+D����������C�Ƕ�����̼������������Һ��Ӧ����Ӧ�����ӷ���ʽΪCO2+2OH-=CO32-+H2O��
����ӦA+D����������C�Ƕ�����̼������������Һ��Ӧ����Ӧ�����ӷ���ʽΪCO2+2OH-=CO32-+H2O��
�ʴ�Ϊ��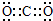 ��CO2+2OH-=CO32-+H2O��
��CO2+2OH-=CO32-+H2O��
��2����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ����о�ˮ���ã�A�Ͳ�ͬ���ļӦ���ɲ�ͬ�����ʣ���A�����Σ�A�еĽ���Ϊ���������ڱ��е������ڣ���A�����κ���ǿ�Ӧ����ƫ�����Σ��������Ӧ����������������Ӧ A+D��B�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O����Al3++4OH-=[Al��OH��4]-��
�ʴ�Ϊ����������A��Al��OH��3+OH-=AlO2-+2H2O ��Al3++4OH-=[Al��OH��4]-��
��3����AΪ�������ʣ�D��ijǿ���ϡ��Һ��A��D��Ӧʱ��A������ͬ�����ﲻͬ����A�DZ�۽�������A�dz�����������AΪ����B�����Σ�C�������Σ�DΪϡ���ᣬ��ӦC+D��B�����ӷ���ʽΪ3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
�ʴ�Ϊ��3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
��4����AΪǿ�DΪ��̬�������Ϊ������Ⱦ��֮һ���ж�DΪSO2��BΪ���������Σ�����ʱ����B��ˮ��Һ¶���ڿ����У�ͼ2��pH�仯�ж�Ϊ������ǿ��pH��С��������HSO3-�����������Ľ������ӦΪ2HSO3-+O2=2H++2SO42-����ͼ2������ʵ��˵����������Ϊ��ԭ�����������ͼ2��y��7��B����ɫ��ӦΪ��ɫ��˵��������Ԫ�أ���B�����������ƣ�A���������ƣ�C���������ƣ�������������ǿ��������ʽ�Σ�������������ӵĵ���̶ȴ���ˮ��̶ȣ������Ӳ�ˮ�⣬���������������ˮ�⣬������������Ӻ�ˮ������������ӣ�������Һ������Ũ�ȴ�С˳����c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ��2HSO3-+O2=2H++2SO42-��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��������1����AΪǿ�DΪ��̬�������Ϊ�����еijɷ֣��ͼӦ˵����CO2���������ӷ���ʽ����д������д��
��2����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ�A�Ͳ�ͬ���ļӦ���ɲ�ͬ�����ʣ���A�����Σ����κ���ǿ�Ӧ����ƫ�����Σ��������Ӧ���������������������ӷ���ʽ����д������д��
��3����AΪ�������ʣ�D��ijǿ���ϡ��Һ��A��D��Ӧʱ��A������ͬ�����ﲻͬ����A�DZ�۽�������A�dz���������Ϊ�����������ӷ���ʽ����д������д��
��4����AΪǿ�DΪ��̬�������Ϊ������Ⱦ��֮һ���ƶ�DΪSO2��BΪHSO3-������ʱ����B��ˮ��Һ¶���ڿ����У���ͼ2������ʵ��˵��������������Ϊ��ԭ�����������������PH��С��ͼ2��y��7��B����ɫ��ӦΪ��ɫ��˵��������Ԫ�أ���B�����������ƣ�A���������ƣ�C���������ƣ�������������ǿ�������Σ�����ˮ���ʹ��Һ�����ԣ�
���������⿼��������Ũ�ȴ�С�ıȽϡ�Ԫ�ػ���������ʵȣ�ע��Ԫ�ػ������������Ӧ�ǽⱾ��ؼ����ѶȲ���
 ����ӦA+D����������C�Ƕ�����̼������������Һ��Ӧ����Ӧ�����ӷ���ʽΪCO2+2OH-=CO32-+H2O��
����ӦA+D����������C�Ƕ�����̼������������Һ��Ӧ����Ӧ�����ӷ���ʽΪCO2+2OH-=CO32-+H2O���ʴ�Ϊ��
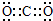 ��CO2+2OH-=CO32-+H2O��
��CO2+2OH-=CO32-+H2O����2����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ����о�ˮ���ã�A�Ͳ�ͬ���ļӦ���ɲ�ͬ�����ʣ���A�����Σ�A�еĽ���Ϊ���������ڱ��е������ڣ���A�����κ���ǿ�Ӧ����ƫ�����Σ��������Ӧ����������������Ӧ A+D��B�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O����Al3++4OH-=[Al��OH��4]-��
�ʴ�Ϊ����������A��Al��OH��3+OH-=AlO2-+2H2O ��Al3++4OH-=[Al��OH��4]-��
��3����AΪ�������ʣ�D��ijǿ���ϡ��Һ��A��D��Ӧʱ��A������ͬ�����ﲻͬ����A�DZ�۽�������A�dz�����������AΪ����B�����Σ�C�������Σ�DΪϡ���ᣬ��ӦC+D��B�����ӷ���ʽΪ3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
�ʴ�Ϊ��3Fe2++4H++NO3-=3Fe3++NO��+2H2O��
��4����AΪǿ�DΪ��̬�������Ϊ������Ⱦ��֮һ���ж�DΪSO2��BΪ���������Σ�����ʱ����B��ˮ��Һ¶���ڿ����У�ͼ2��pH�仯�ж�Ϊ������ǿ��pH��С��������HSO3-�����������Ľ������ӦΪ2HSO3-+O2=2H++2SO42-����ͼ2������ʵ��˵����������Ϊ��ԭ�����������ͼ2��y��7��B����ɫ��ӦΪ��ɫ��˵��������Ԫ�أ���B�����������ƣ�A���������ƣ�C���������ƣ�������������ǿ��������ʽ�Σ�������������ӵĵ���̶ȴ���ˮ��̶ȣ������Ӳ�ˮ�⣬���������������ˮ�⣬������������Ӻ�ˮ������������ӣ�������Һ������Ũ�ȴ�С˳����c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ��2HSO3-+O2=2H++2SO42-��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��������1����AΪǿ�DΪ��̬�������Ϊ�����еijɷ֣��ͼӦ˵����CO2���������ӷ���ʽ����д������д��
��2����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ�A�Ͳ�ͬ���ļӦ���ɲ�ͬ�����ʣ���A�����Σ����κ���ǿ�Ӧ����ƫ�����Σ��������Ӧ���������������������ӷ���ʽ����д������д��
��3����AΪ�������ʣ�D��ijǿ���ϡ��Һ��A��D��Ӧʱ��A������ͬ�����ﲻͬ����A�DZ�۽�������A�dz���������Ϊ�����������ӷ���ʽ����д������д��
��4����AΪǿ�DΪ��̬�������Ϊ������Ⱦ��֮һ���ƶ�DΪSO2��BΪHSO3-������ʱ����B��ˮ��Һ¶���ڿ����У���ͼ2������ʵ��˵��������������Ϊ��ԭ�����������������PH��С��ͼ2��y��7��B����ɫ��ӦΪ��ɫ��˵��������Ԫ�أ���B�����������ƣ�A���������ƣ�C���������ƣ�������������ǿ�������Σ�����ˮ���ʹ��Һ�����ԣ�
���������⿼��������Ũ�ȴ�С�ıȽϡ�Ԫ�ػ���������ʵȣ�ע��Ԫ�ػ������������Ӧ�ǽⱾ��ؼ����ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
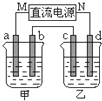 ��ͼ��ʾ��װ���У��ס������ձ��зֱ�ʢ����������CuSO4��Һ��100g 10.00%��K2SO4��Һ��a��b��c��d��Ϊʯī�缫����ͨ��Դһ��ʱ��������K2SO4��ҺŨ��Ϊ10.47%������a�缫���������ӣ�����˵����ȷ���ǣ�������
��ͼ��ʾ��װ���У��ס������ձ��зֱ�ʢ����������CuSO4��Һ��100g 10.00%��K2SO4��Һ��a��b��c��d��Ϊʯī�缫����ͨ��Դһ��ʱ��������K2SO4��ҺŨ��Ϊ10.47%������a�缫���������ӣ�����˵����ȷ���ǣ�������| A���ס�����Һ��pH����С | B���缫b��������������ԼΪ2.8L����״���£� | C���缫d�Ϸ����ķ�ӦΪ��2H2O+2e-?H2��+2OH- | D����ʹ���е���Һ�ָ���ԭ����Ũ�ȣ��ɼ���24.5g��Cu��OH��2 |
 A��B��C��D��Ϊ�������ʣ��֮��Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ����������Լ���Ӧ��������ȥ����
A��B��C��D��Ϊ�������ʣ��֮��Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ����������Լ���Ӧ��������ȥ����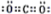
 A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�
A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�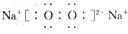
 A��B��C��D��Ϊ������Ԫ�أ�A��B��ͬ�������ڵ�����Ԫ�أ�A��C��ͬ�������ڵ�����Ԫ�أ�A��B��C����Ԫ�ص�ԭ������֮��Ϊ31��DԪ����A��B��C����Ԫ�ؼȲ���ͬ���ڣ�Ҳ��ͬ���壮��ش�
A��B��C��D��Ϊ������Ԫ�أ�A��B��ͬ�������ڵ�����Ԫ�أ�A��C��ͬ�������ڵ�����Ԫ�أ�A��B��C����Ԫ�ص�ԭ������֮��Ϊ31��DԪ����A��B��C����Ԫ�ؼȲ���ͬ���ڣ�Ҳ��ͬ���壮��ش�
 NH3?H2O+H+
NH3?H2O+H+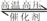 2NH3
2NH3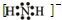
 ���ͱ����γ����ֻ�����X��Y��X��ˮ��Ӧ������һ�־��л�ԭ�ԵĶ�Ԫ��M��1mol�����������ɻ�������Z�����õ�Z����ˮ��Ӧ�IJ���W����12mol KOH������ȫ�кͣ�������������ȼ�����ɻ�����N��N��ˮ��Ӧ����W��DԪ�ص���̬�⻯��Իش��������⣺
���ͱ����γ����ֻ�����X��Y��X��ˮ��Ӧ������һ�־��л�ԭ�ԵĶ�Ԫ��M��1mol�����������ɻ�������Z�����õ�Z����ˮ��Ӧ�IJ���W����12mol KOH������ȫ�кͣ�������������ȼ�����ɻ�����N��N��ˮ��Ӧ����W��DԪ�ص���̬�⻯��Իش��������⣺