��Ŀ����
�˹��̵���ָ����Ԫ��������̬ת��Ϊ����̬�Ĺ��̡�
I.���һЩ��ѧ���о����ø����ӵ����Ե�SCY�մɣ��ܴ���H+��ʵ�鵪�Ĺ̶�һ��ⷨ�ϳɰ����������˵�����������ת���ʡ��ܷ�ӦʽΪ��N2(g)��3H2(g) 2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��
2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��
II.�ݱ�������һ�������£�N2�ڲ��������������Ķ������Ѵ�����������ˮ������Ӧ����Ҫ����ΪNH3����Ӧ�ķ�Ӧ����ʽΪ��2N2(g)��6H2O(g) 4NH3(g)��3O2(g) ��H��Q��
4NH3(g)��3O2(g) ��H��Q��
��1��������Ӧ��ƽ�ⳣ������ʽΪ_______________��
��2��ȡ��ݵ����N2��H2O�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ���������ͬ�ĺ����ܱ������У����¶Ȳ���ͬ������·�����Ӧ����Ӧ��ͬʱ���õ������������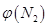 �뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������
�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������
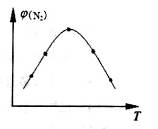
��3����������Ӧ���д���������·���������ͼ��ʾ��a��b��c��d���������У��ܱ�ʾ��Ӧ��ϵ�����仯����_______��ѡ����ĸ���ţ���ͼ�С�H�ľ���ֵΪ1530kJ��mol-1��
III.Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�N2(g)��3H2(g) 2NH3(g) ��H����93.0kJ/mol��
2NH3(g) ��H����93.0kJ/mol��
�ش��������⣺
��1�����II�е����ݣ���O2(g)��2H2(g)��2H2O(g)�ġ�H��______________��
��2����һ���¶��£���1molN2��3mol H2����������������ܱ������з�����Ӧ���ﵽƽ��״̬ʱ��������������ʵ���Ϊ2.8mol��
�ٴ�ƽ��ʱ��H2��ת���ʦ�1��______________��
������ͬ�����£�����ʼʱֻ��NH3���ڸ������У��ﵽƽ��״̬ʱNH3��ת����Ϊ��2������1����2��1ʱ������ʼʱNH3�����ʵ���n(NH3)��_____________mol��
I.���һЩ��ѧ���о����ø����ӵ����Ե�SCY�մɣ��ܴ���H+��ʵ�鵪�Ĺ̶�һ��ⷨ�ϳɰ����������˵�����������ת���ʡ��ܷ�ӦʽΪ��N2(g)��3H2(g)
 2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��
2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��II.�ݱ�������һ�������£�N2�ڲ��������������Ķ������Ѵ�����������ˮ������Ӧ����Ҫ����ΪNH3����Ӧ�ķ�Ӧ����ʽΪ��2N2(g)��6H2O(g)
 4NH3(g)��3O2(g) ��H��Q��
4NH3(g)��3O2(g) ��H��Q����1��������Ӧ��ƽ�ⳣ������ʽΪ_______________��
��2��ȡ��ݵ����N2��H2O�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ���������ͬ�ĺ����ܱ������У����¶Ȳ���ͬ������·�����Ӧ����Ӧ��ͬʱ���õ������������
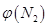 �뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������
�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������
��3����������Ӧ���д���������·���������ͼ��ʾ��a��b��c��d���������У��ܱ�ʾ��Ӧ��ϵ�����仯����_______��ѡ����ĸ���ţ���ͼ�С�H�ľ���ֵΪ1530kJ��mol-1��

III.Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�N2(g)��3H2(g)
 2NH3(g) ��H����93.0kJ/mol��
2NH3(g) ��H����93.0kJ/mol���ش��������⣺
��1�����II�е����ݣ���O2(g)��2H2(g)��2H2O(g)�ġ�H��______________��
��2����һ���¶��£���1molN2��3mol H2����������������ܱ������з�����Ӧ���ﵽƽ��״̬ʱ��������������ʵ���Ϊ2.8mol��
�ٴ�ƽ��ʱ��H2��ת���ʦ�1��______________��
������ͬ�����£�����ʼʱֻ��NH3���ڸ������У��ﵽƽ��״̬ʱNH3��ת����Ϊ��2������1����2��1ʱ������ʼʱNH3�����ʵ���n(NH3)��_____________mol��
��. ����1�֣� N2��6H����6e��===2NH3 ��2�֣�
��.��1��K��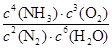 ��2�֣���2������2�֣� ��3��d��2�֣�
��2�֣���2������2�֣� ��3��d��2�֣�
��. ��1����572.0 kJ��mol��1��2�֣���λ��д�����֣� ��2����60%��2�֣� ��2��1�֣�
��.��1��K��
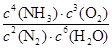 ��2�֣���2������2�֣� ��3��d��2�֣�
��2�֣���2������2�֣� ��3��d��2�֣���. ��1����572.0 kJ��mol��1��2�֣���λ��д�����֣� ��2����60%��2�֣� ��2��1�֣�
�����������.����������ʧȥ���ӷ���������Ӧ�������õ����ӣ�������ԭ��Ӧ�����Դ��ܷ�Ӧ�����жϳ�H2�DZ�������Ӧ��������ͨ�룬N2������������ԭ��Ӧ��������ͨ�룬�缫��Ӧʽ��N2+ 6H++6e-��2NH3��
��.��1����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը��ݷ�Ӧ����ʽ��֪���÷�Ӧ��ƽ�ⳣ���ı���ʽK��
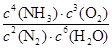 ��
����2������������ͬʱ���ڵ���������������¶ȱ仯�Ĺ�ϵ�����ߵı仯��������С���¶����߷�Ӧ���ʼӿ졣�ڴ����У���Ӧ��ͬʱ�䣬�����µ�������δ�ﵽƽ������㣬���¶ȵ����ߣ�����������������¶����߶�������ߵ���ߵ���ij�¶��´ﵽƽ��ĵ㣬֮������㣬����ƽ���ĵ�������������������¶����߶����ͣ���˵�������¶�ƽ��������Ӧ�����ƶ���������ɵ����ķ�Ӧ�Ƿ��ȷ�Ӧ�����˷�Ӧ������ӦΪ���ȷ�Ӧ��
��3�����ݣ�2���Ľ��ۣ���Ӧ�����ȷ�Ӧ��Ӧ��c��d�������Ҵ𰸡�ʹ�ô������Խ��ͷ�Ӧ�Ļ�ܣ����Ӧ��ѡd��
��.��1����Ӧ��N2(g)��3H2(g)
 2NH3(g)�ͷ�Ӧ��2N2(g)��6H2O(g)
2NH3(g)�ͷ�Ӧ��2N2(g)��6H2O(g) 4NH3(g)��3O2(g)����������ݸ�˹���ɿ�֪���١�
4NH3(g)��3O2(g)����������ݸ�˹���ɿ�֪���١�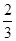 ���ڡ�
���ڡ�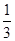 ���õ���ӦO2(g)��2H2(g)��2H2O(g)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H��2/3������93 kJ��mol��1����1/3��1530 kJ��mol��1����572 kJ��mol��1��
���õ���ӦO2(g)��2H2(g)��2H2O(g)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H��2/3������93 kJ��mol��1����1/3��1530 kJ��mol��1����572 kJ��mol��1����2������N2(g)��3H2(g)
 2NH3(g)��֪��4mol�ķ�Ӧ����ȫ��Ӧ�������ʵ�����С2mol����˵����٣�4��2.8��mol��1.2molʱ���μӷ�Ӧ��H2Ϊ1.8mol������������ת����Ϊ
2NH3(g)��֪��4mol�ķ�Ӧ����ȫ��Ӧ�������ʵ�����С2mol����˵����٣�4��2.8��mol��1.2molʱ���μӷ�Ӧ��H2Ϊ1.8mol������������ת����Ϊ ��100%��60����
��100%��60�������谱�������ʵ�����x����1����2��1�����Ԧ�2��40%��ƽ��ʱ���������ʵ�����1.2mol���������İ�����x��1.2mol����x��40%��x��1.2mol�����x��2.0mol��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CO2(g)��H2(g)��Ӱ�죬�ҵ�ѹǿ�ȼ�ѹǿ��
CO2(g)��H2(g)��Ӱ�죬�ҵ�ѹǿ�ȼ�ѹǿ��
 2C(g)+6D�ڲ�ͬѹǿ��B��(B���������)��ʱ��ı仯����Dһ��������
2C(g)+6D�ڲ�ͬѹǿ��B��(B���������)��ʱ��ı仯����Dһ�������� H2NCOONH4(���������)(l) ��H1
H2NCOONH4(���������)(l) ��H1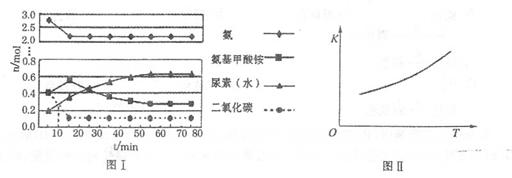
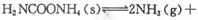
 ��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
 Si3N4��s��+6CO��g��
Si3N4��s��+6CO��g��