��Ŀ����
����Ŀ����ҵ�����õ�ⱥ��ʳ��ˮ����ȡ�ռ���õ�ʳ��ˮ�����ξ��ơ���һ�ξ�����Ҫ���ó�������ȥ����ˮ��Ca2+��Mg2+��Fe3+��SO42-�����ӣ��������£�
��.�����ˮ�м������BaCl2��Һ�����ˣ�
��.��������Һ�м������Na2CO3��Һ�����ˣ�
��.�����������Һ��pH�����һ�ξ�����ˮ��
(1)���̢��г�ȥ��������______��
(2)���ǹ��̢������ɵIJ��ֳ���������20��ʱ���ܽ��(g/100 gH2O)��
CaSO4 | Mg2(OH)2CO3 | CaCO3 | BaSO4 | BaCO3 | Fe(OH)3 |
2.6��10-2 | 2.5��10-4 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 | 4.8��10-9 |
���ñ�����Ϣ�ش��������⣺
�ٹ��̢������ɵ���Ҫ������CaCO3��Mg2(OH)2CO3���______��
�ڹ��̢�ѡ�õ���BaCl2����ѡ��CaCl2��ԭ����______��
�۳�ȥMg2+�����ӷ���ʽ��______��
�ܼ��Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ�ԭ����______��
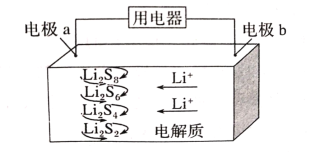
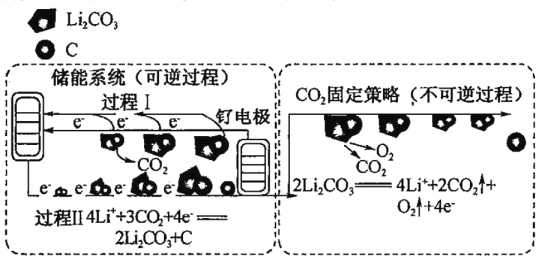
(3)�ڶ��ξ���Ҫ��ȥ����I-��IO3-��NH4+��Ca2+��Mg2+������ʾ��ͼ��ͼ��

�ٹ��̢���ȥ��������______��
����ˮb�к���SO42-��Na2S2O3��IO3-��ԭΪI2�����ӷ���ʽ��______��
���ڹ��̢������õ�Na2S2O3�׳ƺ�������һ����Ҫ�Ļ���ԭ�ϡ���Ʒ������Ҫ�ɷ���Na2S2O3��5H2OΪ�˲ⶨ�京Na2S2O3��5H2O�Ĵ��ȣ���ȡ8.00 g��Ʒ�����Ƴ�250.0 mL��Һ��ȡ25.00 mL����ƿ�У��μӵ�����Һ��ָʾ��������Ũ��Ϊ0.0500 mol/L�ĵ�ˮ�ζ�(������Ӧ2S2O32-+I2=S4O62-+2I-)���ζ��ﵽ�յ�ʱ��������______���±���¼�ζ������
�ζ����� | �ζ�ǰ����(mL) | �ζ��ζ������(mL) |
��һ�� | 0.30 | 31.12 |
�ڶ��� | 0.36 | 31.56 |
������ | 1.10 | 31.88 |
������Ʒ�Ĵ���Ϊ______��
���𰸡�SO42- BaCO3��Fe(OH)3 BaSO4���ܽ��С��CaSO4 2Mg2++2CO32-+H2O= Mg2(OH)2CO3��+CO2�� ̼�ᱵ���ܽ������������ӳ�����ȫ����˵��þ���Ӻ�����Ҳ������ȫ NH4+��I- 5S2O32-+8IO3-+H2O=10SO42-+4I2+2H+ ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ 95.48%
��������
��һ�ξ��ƣ������ˮ�м������BaCl2��Һ��Ba2+��SO42-�õ�BaSO4���������ˣ���ȥSO42-����������Һ�м������Na2CO3��Һ����ȥCa2+��Mg2+��Fe3+������Ba2+���õ����ˣ����������pH����ȥ������̼���ƣ��õ�������ˮ��
(1)����Ba2+��SO42-�ķ�Ӧ�ɵã�
(2)�پ���I������Һ������������Ҫ�ǣ�Ca2+��Mg2+��Fe3+��Ba2+���������Na2CO3��Һ��Ca2+��Ba2+��CO32-����CaCO3��BaCO3��Mg2+��CO32-����Mg2(OH)2CO3��Fe3+��CO32-����˫ˮ������ Fe(OH)3��
�ڳ�������ʱ��������ѡ�������Խ����Խ�ã�
��Mg2+��CO32-����Mg2(OH)2CO3�Ͷ�����̼���ݴ���д��
��Ca2+��Mg2+��Ba2+��CaCO3��Mg2(OH)2CO3��BaCO3����ʽ��ȥ�������ܽ��С�ij�����ȫ���ܽ�ȴ�IJų���������
�ڶ��ξ��ƣ����һ�ξ�����ˮ(��������ΪI-��IO3-��NH4+��Ca2+��Mg2+)�м���NaClO��NaClO���������ԣ���I-������NH4+����ΪN2���ټ���Na2S2O3����IO3-��ԭΪI2�������I2��ͨ�����ӽ�������ȥCa2+��Mg2+�����ʣ����Һ�õ�NaOH��
(3)�ٸ���NaClO�������Է�����
��Na2S2O3��IO3-��ԭΪI2������������ΪSO42-���ݴ���д��
����Ʒ�õ�ˮ�ζ����õ�����Һ��ָʾ��������Ʒ��Ӧ��ȫʱ���������������ɫ�����ݷ�Ӧ�й�ϵʽ��2S2O32-��I2����n(Na2S2O3��5H2O)=2n(I2)�����ݱ�Һ�����ļ���ɵá�
(1)�����ˮ�м������BaCl2��Һ��Ba2+��SO42-�õ�BaSO4���������ˣ���ȥSO42-��
(2)�پ���I������Һ������������Ҫ�ǣ�Ca2+��Mg2+��Fe3+��Ba2+���������Na2CO3��Һ��Ca2+��Ba2+��CO32-����CaCO3��BaCO3��Mg2+��CO32-����Mg2(OH)2CO3��Fe3+��CO32-����˫ˮ������ Fe(OH)3�����Թ��̢������ɵ���Ҫ������CaCO3��Mg2(OH)2CO3���BaCO3��Fe(OH)3��
�ڸ��ݱ���֪��BaSO4���ܽ��С��CaSO4���ʹ��̢�ѡ�õ���BaCl2����ѡ��CaCl2��
��Mg2+��CO32-����Mg2(OH)2CO3��CO2�����ӷ���ʽΪ��2Mg2++2CO32-+H2O= Mg2(OH)2CO3��+CO2����
��Ca2+��Mg2+��Ba2+��CaCO3��Mg2(OH)2CO3��BaCO3����ʽ��ȥ�������ܽ��С�ij�����ȫ���ܽ�ȴ�IJſ�ʼ�������ɱ���֪��̼�ᱵ���ܽ������������ӳ�����ȫ����˵��þ���Ӻ�����Ҳ������ȫ���ʼ��Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ�
(3)��NaClO���������ԣ��ܽ�I-�������������̿�֪NH4
��Na2S2O3��IO3-��ԭΪI2������������ΪSO42-���÷�Ӧ�����ӷ���ʽΪ��5S2O32-+8IO3-+H2O=10SO42-+4I2+2H+��
����Ʒ�õ�ˮ�ζ����õ�����Һ��ָʾ��������Ʒ��Ӧ��ȫʱ���������������ɫ���ʵ���Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵���ﵽ�ζ��յ㣻
���ݱ������ݿ�֪���ڶ�������ƫ��ϴ�����ȡ�õ�һ�顢����������ݣ�������ȥ�ĵ�ˮ���Ϊ��V(��ˮ)=![]() mL=30.80 mL��
mL=30.80 mL��
���ݷ�Ӧ�й�ϵʽ��2S2O32-��I2����n(Na2S2O35H2O)=2n(I2)������Ʒ������Na2S2O35H2O�����ʵ���n(Na2S2O35H2O)=2��0.0500 mol/L��30.80��10-3L��![]() =0.0308 mol����Ʒ������Na2S2O35H2O�Ĵ���Ϊ��
=0.0308 mol����Ʒ������Na2S2O35H2O�Ĵ���Ϊ��![]() ��100%=95.48%��
��100%=95.48%��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�����Ŀ��ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
(1)ʵ�����ϱ��д�С�����ձ�����ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β����������50 mL 0.50mol/L���ᡢ50 mL 0.55mol/LNaOH��Һ��ʵ����ȱ�ٵIJ�����Ʒ��____��____��
(2)NaOH�Թ�����ԭ����_______��
(3)ʵ����������60 mL 0.50 mol/L�����50 mL 0.55 mol/LNaOH��Һ����������Ӧ��������ʵ����ȣ����ų�������_______(���ȡ�����ȡ�)�������к���____(���ȡ�����ȡ�)��������________��
(4)���Ǽ�¼��ʵ���������£�
ʵ �� �� Ʒ | ��Һ�¶� | ||
t1 | t2 | ||
�� | 50 mL 0.55mol/LNaOH��Һ | 20 �� | 23.3 �� |
50 mL 0.50mol/L���� | |||
�� | 50 mL 0.55mol/LNaOH��Һ | 20 �� | 23.5 �� |
50 mL 0.50mol/L���� | |||
��֪��Q=cm(t2-t1)����Ӧ����Һ�ı�����cΪ4.18 J/(��g)�������ʵ��ܶȾ�Ϊ1 g/cm3������ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ��____��
(5)���õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к���(��H)______(�ƫ��ƫС�����䡱)����ԭ����_______��
����Ŀ��ijУ��ȤС���ͬѧΧ��Fe3+��S2-�ķ�Ӧ�������˼��ҵ����磬����������������Ӧ�ļ�¼
(1)��ͬѧ��Ϊ��������Fe3+��I-��Fe3+��S2-Ҳ�ܷ���������ԭ��Ӧ�����У���ͬѧ�ᵽ��Fe3+��I-������Ӧ�����ӷ���ʽ��______��
(2)��ͬѧ��Ϊ��ͬѧ����Ƿ�ܣ�����ΪFe3+��S2-������Ӧ�����ӷ���ʽ���ܸ��μ�˳���йء���������Ȼ�����Һ����μ���������Һ�����ӷ���ʽ������(1)�е����ӷ���ʽ���������������Һ����μ����Ȼ�����Һ�����ӷ���ʽ��������ͬ��Ӧ��Ϊ______��
(3)��ͬѧ�Ŀ�����ף�����λͬѧ����ͬ����Ȼ��ȣ�������������![]() ��
��![]() �ķ�Ӧ��ȡ�����˵������ͬѧԤ�ڵ�Fe3+��S2-������Ӧ�����ӷ���ʽӦΪ______��
�ķ�Ӧ��ȡ�����˵������ͬѧԤ�ڵ�Fe3+��S2-������Ӧ�����ӷ���ʽӦΪ______��
(4)������ͼ���λͬѧ���ˣ���Լ������ʵ���ҡ����ж�ͬѧ����������ʵ�飺��ȡ______gFeCl3���������ձ��У���______�ܽ⣬�ټ�����ˮ���100 mL��Һ���á�ȡ2 mL1 mol/L��FeCl3��Һ���Թ��У���εμ�0.1 mol/L��Na2S��Һ����ʼ�ֲ����������ĺ�ɫ����������ɫ����������ʧ(��������ɫ����������ʧ)��ͬʱ��Һ��dz��ɫ���ǣ����������ĺ��ɫ��״�����������ŵ����ij�������ζ���μӵ�5��Na2S��Һʱ����Һ��ʼ��������������ɫ����������������ʧ�������μ�Na2S��Һ������������������ͬѧ������ʵ�����ͬѧ������ʵ��ֻ�ڵμ�˳���ϴ��ڲ�ͬ���պ��γɲ��䡣������ͬѧ��ʵ�����______��
(5)��ͬѧ����ͬѧ��ʵ������еõ��ĺ�ɫ����A����ͬʵ�������е�FeSҩƷ(������)һ����������Сʵ�顣
ʵ������ | ʵ������ |
��A+H2O | ��������������������ڣ�����Ҳ���ܽ⣬��Һ��ɫҲ���ı䣬��û�г�������ζ���������� |
��A+HCl | ��ɫ�����ܽ⣬�����ִ���dz��ɫ�����������г�������ζ���������� |
��FeS+HCl | ���ɴ�����������ζ�����壬��û��dz��ɫ�������� |
������ͬѧ��ʵ���֪����ɫ����A����Ϊ______![]() �ѧʽ
�ѧʽ![]() ���������Ի������Һ�����ȶ����ڡ��ݴ˿�֪��������Һ�м��������Ȼ�����Һ�����ӷ���ʽ����Ϊ______��
���������Ի������Һ�����ȶ����ڡ��ݴ˿�֪��������Һ�м��������Ȼ�����Һ�����ӷ���ʽ����Ϊ______��