Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņ“Ý «“Ľ÷÷‘ŕĻ§“Ķ°Ę…ķĽÓ…Ō”–Ļ„∑ļ”√ÕĺĶńĹū Ű°£
“—÷™£ļĘŔĹū Ű‘ŕňģ÷–īś‘ŕ»ÁŌ¬»‹Ĺ‚∆Ĺļ‚Ļż≥Ő£ļM![]() Mx+ + xe-£¨
Mx+ + xe-£¨
«‚∆Ý‘ŕňģ»‹“ļ÷–“≤īś‘ŕ»ÁŌ¬∆Ĺļ‚Ļż≥Ő£ļH2![]() 2H++ 2e-
2H++ 2e-
ĘŕAg2S ĶńKsp=6.7°Ń10-50£Ľ AgClĶńKsp=1.6°Ń10-10
ĺ›īňĽōīūŌ¬Ń–”–Ļōő Ő‚£ļ
£®1£©“Ý÷ ≤ÕĺŖŅ……ĪĺķŌŻ∂ĺ£¨‘≠“Ú «_________________£®”√ ĶĪĶń∑Ĺ≥Ő ĹļÕőń◊÷ĪŪ ŲňĶ√ų£©£ĽĹū Ű“Ý”Ž«‚ŃÚňŠŅ…∑ī”¶…ķ≥…ļŕ…ęĻŐŐŚļÕőř…ę∆ÝŐŚ£¨–ī≥Ųł√∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ_______________
£®2£©Ĺū Ű“Ý”ŽŌűňŠ“Ý»‹“ļ◊ť≥…ĶÁ≥ō ĺ“‚Õľ»Á”“£¨aĶÁľęĶń∑ī”¶ő™________________£¨NO3-ī”ĶÁ≥ō________≤ŗ»‹“ļŌÚĶÁ≥ō_______≤ŗ»‹“ļ“∆∂Į £®ŐÓ°į◊ů°ĪĽÚ°į”“°Ī£©°£

£®3£©ŌűňŠ“ÝľŻĻ‚ĽÚ ‹»»“◊∑÷Ĺ‚ő™Ag°ĘNO2°ĘO2£¨∑ī”¶÷–…ķ≥…NO2°ĘO2Ķń őÔ÷ ĶńŃŅ÷ģĪ»ő™___________£¨Ĺ꼞ļŌ∆ÝŐŚÕ®ĻżňģőŁ ’ļů£¨ £”ŗ∆ÝŐŚő™________________
£®4£©“—÷™£ļAg+(aq) + 2NH3H2O(aq)![]() [Ag(NH3)2]+(aq) + 2H2O K=1.6°Ń107£¨–ī≥ŲAgCl»‹”ŕįĪňģĶńņŽ◊”∑Ĺ≥Ő Ĺ________________________£Ľľ∆ň„ł√∑ī”¶Ķń∆Ĺļ‚≥£ żK=___________°£‘଻ĽĮ“Ý»‹”ŕįĪňģļůĶń»‹“ļ÷–Ķőľ”Ō°ŌűňŠ£¨ĽŠ‘Ŕ≤ķ…ķį◊…ꬻĽĮ“Ý≥ŃĶŪ£¨Ķőľ”ŌűňŠ÷Ńł’ļ√≥ŃĶŪÕÍ»ę£¨»°…Ō≤„«Ś“ļ≤‚∆špH£¨∑ĘŌ÷≥ ňŠ–‘£¨÷ų“™‘≠“Ú «_______________£®”√ņŽ◊”∑Ĺ≥Ő ĹĪŪ ĺ£©°£
[Ag(NH3)2]+(aq) + 2H2O K=1.6°Ń107£¨–ī≥ŲAgCl»‹”ŕįĪňģĶńņŽ◊”∑Ĺ≥Ő Ĺ________________________£Ľľ∆ň„ł√∑ī”¶Ķń∆Ĺļ‚≥£ żK=___________°£‘଻ĽĮ“Ý»‹”ŕįĪňģļůĶń»‹“ļ÷–Ķőľ”Ō°ŌűňŠ£¨ĽŠ‘Ŕ≤ķ…ķį◊…ꬻĽĮ“Ý≥ŃĶŪ£¨Ķőľ”ŌűňŠ÷Ńł’ļ√≥ŃĶŪÕÍ»ę£¨»°…Ō≤„«Ś“ļ≤‚∆špH£¨∑ĘŌ÷≥ ňŠ–‘£¨÷ų“™‘≠“Ú «_______________£®”√ņŽ◊”∑Ĺ≥Ő ĹĪŪ ĺ£©°£
°ĺīūįł°ŅAg ![]() Ag++ e-£¨Ag»‹Ĺ‚ Õ∑ŇĶńAg+ «÷ōĹū Ű—ŰņŽ◊”£¨ ĻŌłĺķŐŚńŕĶįį◊÷ Īš–‘£¨ī”∂Ý…ĪĺķŌŻ∂ĺ 2Ag + H2S = Ag2S + H2°Ł Ag++ e-= Ag ◊ů ”“ 2:1 —ű∆Ý AgCl + 2NH3H2O(aq)
Ag++ e-£¨Ag»‹Ĺ‚ Õ∑ŇĶńAg+ «÷ōĹū Ű—ŰņŽ◊”£¨ ĻŌłĺķŐŚńŕĶįį◊÷ Īš–‘£¨ī”∂Ý…ĪĺķŌŻ∂ĺ 2Ag + H2S = Ag2S + H2°Ł Ag++ e-= Ag ◊ů ”“ 2:1 —ű∆Ý AgCl + 2NH3H2O(aq)![]() [Ag(NH3)2]+(aq) + Cl-+ 2H2O K=2.56°Ń10-3 NH4++ H2O
[Ag(NH3)2]+(aq) + Cl-+ 2H2O K=2.56°Ń10-3 NH4++ H2O![]() NH3H2O + H+
NH3H2O + H+
°ĺĹ‚őŲ°Ņ
£®1£©łýĺ›Ő‚łÝ–ŇŌĘ£ļM![]() Mx+ + xe-£¨Ag ß»•ĶÁ◊”…ķ≥…ĶńAg+ «÷ōĹū ŰņŽ◊”£¨Ņ…“‘ ĻĶįį◊÷ Īš–‘£¨ī”∂ÝŅ…“‘…ĪĺķŌŻ∂ĺ£ĽĹū Ű“Ý”Ž«‚ŃÚňŠŅ…∑ī”¶…ķ≥…ļŕ…ęĻŐŐŚAg2SļÕőř…ę∆ÝŐŚH2£Ľ
Mx+ + xe-£¨Ag ß»•ĶÁ◊”…ķ≥…ĶńAg+ «÷ōĹū ŰņŽ◊”£¨Ņ…“‘ ĻĶįį◊÷ Īš–‘£¨ī”∂ÝŅ…“‘…ĪĺķŌŻ∂ĺ£ĽĹū Ű“Ý”Ž«‚ŃÚňŠŅ…∑ī”¶…ķ≥…ļŕ…ęĻŐŐŚAg2SļÕőř…ę∆ÝŐŚH2£Ľ
£®2£©Ĺū Ű“Ý”ŽŌűňŠ“Ý»‹“ļ◊ť≥…ĶńŇ®≤ÓĶÁ≥ō£¨aĶÁľę «’żľę£¨Ag+Ķ√ĶĹĶÁ◊”…ķ≥…Ag£¨bĶÁľę «łļľę£¨Ag ß»•ĶÁ◊”…ķ≥…Ag+£¨“űņŽ◊”“∆ŌÚłļľę£Ľ
£®3£©ŌűňŠ“ÝľŻĻ‚ĽÚ ‹»»“◊∑÷Ĺ‚ő™Ag°ĘNO2°ĘO2£¨łý囑≠◊” ōļ„Ņ…»∑∂®∑ī”¶÷–…ķ≥…NO2°ĘO2ĶńőÔ÷ ĶńŃŅ÷ģĪ»£Ľłýĺ›![]() £¨Ņ…ľ∆ň„ĹęőÔ÷ ĶńŃŅ÷ģĪ»ő™2:1ĶńNO2ļÕO2ĽžļŌ∆ÝŐŚÕ®ĻżňģőŁ ’ļů£¨ £”ŗ∆ÝŐŚĶń≥…∑÷£Ľ
£¨Ņ…ľ∆ň„ĹęőÔ÷ ĶńŃŅ÷ģĪ»ő™2:1ĶńNO2ļÕO2ĽžļŌ∆ÝŐŚÕ®ĻżňģőŁ ’ļů£¨ £”ŗ∆ÝŐŚĶń≥…∑÷£Ľ
£®4£©łýĺ›Ag+(aq) + 2NH3H2O(aq)![]() [Ag(NH3)2]+(aq) + 2H2O£¨–ī≥ŲAgCl»‹”ŕįĪňģĶńņŽ◊”∑Ĺ≥Ő Ĺ£Ľłý図Ý≥ŲĶń∑ī”¶ĶńKļÕAgClĶńKsp£¨Ņ…ľ∆ň„AgCl»‹”ŕįĪňģĶń∑ī”¶Ķń∆Ĺļ‚≥£ ż°£‘଻ĽĮ“Ý»‹”ŕįĪňģļůĶń»‹“ļ÷–Ķőľ”Ō°ŌűňŠ∑ī”¶ļůĶń…Ō≤„«Ś“ļ÷–ļ¨NH4+£¨ňģĹ‚≥ ňŠ–‘°£
[Ag(NH3)2]+(aq) + 2H2O£¨–ī≥ŲAgCl»‹”ŕįĪňģĶńņŽ◊”∑Ĺ≥Ő Ĺ£Ľłý図Ý≥ŲĶń∑ī”¶ĶńKļÕAgClĶńKsp£¨Ņ…ľ∆ň„AgCl»‹”ŕįĪňģĶń∑ī”¶Ķń∆Ĺļ‚≥£ ż°£‘଻ĽĮ“Ý»‹”ŕįĪňģļůĶń»‹“ļ÷–Ķőľ”Ō°ŌűňŠ∑ī”¶ļůĶń…Ō≤„«Ś“ļ÷–ļ¨NH4+£¨ňģĹ‚≥ ňŠ–‘°£
£®1£©Ag‘ŕňģ÷–īś‘ŕ»ÁŌ¬»‹Ĺ‚∆Ĺļ‚Ļż≥Ő£ļAg![]() Ag++ e-£¨Ag»‹Ĺ‚ Õ∑ŇĶńAg+ «÷ōĹū Ű—ŰņŽ◊”£¨ ĻŌłĺķŐŚńŕĶįį◊÷ Īš–‘£¨ī”∂Ý…ĪĺķŌŻ∂ĺ£ĽĹū Ű“Ý”Ž«‚ŃÚňŠŅ…∑ī”¶…ķ≥…«‚∆ÝļÕŃÚĽĮ“Ý£¨∑Ĺ≥Ő Ĺő™2Ag + H2S = Ag2S + H2°Ł£Ľ
Ag++ e-£¨Ag»‹Ĺ‚ Õ∑ŇĶńAg+ «÷ōĹū Ű—ŰņŽ◊”£¨ ĻŌłĺķŐŚńŕĶįį◊÷ Īš–‘£¨ī”∂Ý…ĪĺķŌŻ∂ĺ£ĽĹū Ű“Ý”Ž«‚ŃÚňŠŅ…∑ī”¶…ķ≥…«‚∆ÝļÕŃÚĽĮ“Ý£¨∑Ĺ≥Ő Ĺő™2Ag + H2S = Ag2S + H2°Ł£Ľ
£®2£©NO3-ī”łŖŇ®∂»«Ý”Ú“∆∂ĮĶĹĶÕŇ®∂»«Ý”Ú£¨ňý“‘NO3-ī”◊ů≤ŗ“∆∂ĮĶĹ”“≤ŗ£Ľaő™’żľę£¨bő™łļľę£Ľaľę∑ī”¶ő™Ag++ e-= Ag£Ľ
£®3£©ŌűňŠ“ÝľŻĻ‚ĽÚ ‹»»“◊∑÷Ĺ‚ő™Ag°ĘNO2°ĘO2£¨Ķ™‘≠◊”£ļ—ű‘≠◊”=1:3£¨…ŤNO2°ĘO2ĶńőÔ÷ ĶńŃŅ∑÷Īūx mol°Ęy mol,ő™łý囑™ňō ōļ„£¨![]() £¨NO2°ĘO2ĶńőÔ÷ ĶńŃŅ÷ģĪ»ő™2:1£Ľłýĺ›
£¨NO2°ĘO2ĶńőÔ÷ ĶńŃŅ÷ģĪ»ő™2:1£Ľłýĺ›![]() £¨Ĺ꼞ļŌ∆ÝŐŚÕ®ĻżňģőŁ ’ļů£¨ £”ŗ∆ÝŐŚő™—ű∆Ý£Ľ
£¨Ĺ꼞ļŌ∆ÝŐŚÕ®ĻżňģőŁ ’ļů£¨ £”ŗ∆ÝŐŚő™—ű∆Ý£Ľ
£®4£©AgCl»‹”ŕįĪňģĶńņŽ◊”∑Ĺ≥Ő ĹAgCl + 2NH3H2O(aq)![]() [Ag(NH3)2]+(aq) + Cl-+ 2H2O£¨
[Ag(NH3)2]+(aq) + Cl-+ 2H2O£¨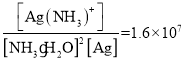 £¨AgClĶńKsp=1.6°Ń10-10£¨ľī
£¨AgClĶńKsp=1.6°Ń10-10£¨ľī![]() £Ľňý“‘
£Ľňý“‘ £Ľ‘଻ĽĮ“Ý»‹”ŕįĪňģļůĶń»‹“ļ÷–Ķőľ”Ō°ŌűňŠ,∑Ę…ķNH3H2O+HNO3= NH4NO3+ H2O£¨ĽŠ ĻAgCl + 2NH3H2O(aq)
£Ľ‘଻ĽĮ“Ý»‹”ŕįĪňģļůĶń»‹“ļ÷–Ķőľ”Ō°ŌűňŠ,∑Ę…ķNH3H2O+HNO3= NH4NO3+ H2O£¨ĽŠ ĻAgCl + 2NH3H2O(aq)![]() [Ag(NH3)2]+(aq) + Cl-+ 2H2O∑ī”¶Ķń∆Ĺļ‚ńśŌÚ“∆∂Į£¨≤ķ…ķį◊…ꬻĽĮ“Ý≥ŃĶŪ£¨Ķőľ”ŌűňŠ÷Ńł’ļ√≥ŃĶŪÕÍ»ę£¨»‹“ļ÷–Ķń»‹÷ ő™NH4NO3£¨ÔßłýņŽ◊”ňģĹ‚£¨NH4++ H2O
[Ag(NH3)2]+(aq) + Cl-+ 2H2O∑ī”¶Ķń∆Ĺļ‚ńśŌÚ“∆∂Į£¨≤ķ…ķį◊…ꬻĽĮ“Ý≥ŃĶŪ£¨Ķőľ”ŌűňŠ÷Ńł’ļ√≥ŃĶŪÕÍ»ę£¨»‹“ļ÷–Ķń»‹÷ ő™NH4NO3£¨ÔßłýņŽ◊”ňģĹ‚£¨NH4++ H2O![]() NH3H2O + H+£¨ňý“‘»‹“ļ≥ ňŠ–‘°£
NH3H2O + H+£¨ňý“‘»‹“ļ≥ ňŠ–‘°£

°ĺŐ‚ńŅ°Ņ—«Ōűű£¬»(ClNO)Ņ…”…NO”ŽCl2‘ŕÕ®≥£ŐűľĢŌ¬∑ī”¶Ķ√ĶĹ£¨ĽĮ—ß∑Ĺ≥Ő Ĺő™2NO(g)£ęCl2(g)![]() 2ClNO(g)°£
2ClNO(g)°£
(1)‘ŕ“Ľ∂®ő¬∂»Ō¬£¨ł√∑ī”¶”ŕ“Ľļ„»›√‹Ī’»›∆ų÷–īÔĶĹ∆Ĺļ‚£¨ľŐ–ÝÕ®»ŽCl2£¨ńś∑ī”¶ňŔ¬ ________(ŐÓ°į‘Ųīů°Ī°įľű–°°ĪĽÚ°į≤ĽĪš°Ī)°£
(2)“—÷™ľł÷÷ĽĮ—ßľŁĶńľŁń‹ żĺ›»ÁĪŪ(—«Ōűű£¬»ĶńĹŠĻĻ Ĺő™Cl°™N=O)£ļ
ĽĮ—ßľŁ | NO | Cl°™Cl | Cl°™N | N=O |
ľŁń‹/(kJ°§mol£≠1) | 630 | 243 | a | 607 |
2NO(g)£ęCl2(g)![]() 2ClNO(g)°°¶§H£Ĺ£≠111 kJ°§mol£≠1£¨‘Úa£Ĺ________°£
2ClNO(g)°°¶§H£Ĺ£≠111 kJ°§mol£≠1£¨‘Úa£Ĺ________°£
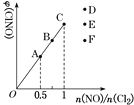
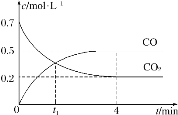
(3)‘ŕ1 LĶńļ„»›√‹Ī’»›∆ų÷–≥š»Ž2 mol NO(g)ļÕ1 mol Cl2(g)£¨‘ŕ≤ĽÕ¨ő¬∂»Ō¬≤‚Ķ√c(ClNO)”Ž ĪľšĶńĻōŌĶ»ÁÕľ£ļ
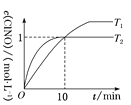
ĘŔī”∑ī”¶Ņ™ ľĶĹ10 min ĪNOĶń∆Ĺĺý∑ī”¶ňŔ¬ v(NO)£Ĺ________mol°§L£≠1°§min£≠1°£
ĘŕT2 Īł√∑ī”¶Ķń∆Ĺļ‚≥£ żK£Ĺ________°£
(4)“Ľ∂®ŐűľĢŌ¬‘ŕļ„ő¬ļ„»›Ķń√‹Ī’»›∆ų÷–įī“Ľ∂®Ī»ņż≥š»ŽNO(g)ļÕCl2(g)£¨∆Ĺļ‚ ĪClNOĶńŐŚĽż∑÷ żňśn(NO)/n(Cl2)ĶńĪšĽĮÕľŌŮ»ÁÕľ£¨‘ÚA°ĘB°ĘC»ż◊īŐ¨÷–£¨NOĶń◊™ĽĮ¬ ◊ÓīůĶń «________Ķ„£¨ĶĪn(NO)/n(Cl2)£Ĺ1.5 Ī£¨∑ī”¶īÔĶĹ∆Ĺļ‚◊īŐ¨ClNOĶńŐŚĽż∑÷ żŅ…ń‹ «D°ĘE°ĘF»żĶ„÷–Ķń________Ķ„°£