ЬтФПФкШн
ЁОЬтФПЁПГЃЮТЯТЃЌИФБф0.1molЁЄL-1ЖўдЊШѕЫсH2AШмвКЕФpHЃЌШмвКжаЕФH2AЁЂHA-ЁЂA2-ЕФЮяжЪЕФСПЗжЪ§![]() (X)ЫцpHЕФБфЛЏШчЭМЫљЪО[вбжЊ
(X)ЫцpHЕФБфЛЏШчЭМЫљЪО[вбжЊ![]() (X) =
(X) =![]() ]ЁЃЯТСаа№ЪіДэЮѓЕФЪЧ( )
]ЁЃЯТСаа№ЪіДэЮѓЕФЪЧ( )
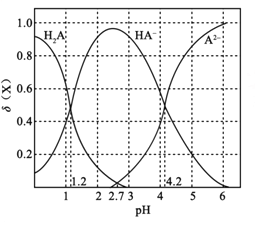
A. pH=1.2ЪБЃЌc(K+)+c(H+)=c(H2A)+c(OH-)
B. ГЃЮТЯТЃЌH2AЕФЕчРыЦНКтГЃЪ§Ka2=10-42
C. pH=2.7ЪБЃЌc(HA-)ЃОc(H2A)=c(A2-)
D. KHAШмвКжаРызгХЈЖШЮЊc(K+)ЃОc(HA-)ЃОc(OH-)ЃОc(H2A)ЃОc(H+)ЃОc(A2-)
ЁОД№АИЁПD
ЁОНтЮіЁП
гЩЭМЯѓПЩжЊЃЌpH=1.2ЪБЃЌcЃЈH2AЃЉ=cЃЈHA-ЃЉЃЌpH=2.7ЪБЃЌcЃЈH2AЃЉ=cЃЈA2-ЃЉЃЌpH=4.2ЪБЃЌcЃЈHA-ЃЉ=cЃЈA2-ЃЉЃЌЫцзХpHЕФдіДѓЃЌcЃЈH2AЃЉж№НЅМѕаЁЃЌcЃЈHA-ЃЉЯШдіДѓКѓМѕаЁЃЌcЃЈA2-ЃЉж№НЅдіДѓЃЌНсКЯЕчРыЦНКтГЃЪ§вдМАЬтИјЪ§ОнМЦЫуЃЌПЩИљОнзнзјБъБШНЯХЈЖШДѓаЁЁЃ
AЯюЁЂгЩЭМЯѓПЩжЊЃЌpH=1.2ЪБЃЌcЃЈH2AЃЉ=cЃЈHA-ЃЉЁЂc(A2-)=0ЃЌдђЕчКЩЪиКуЙиЯЕЮЊcЃЈK+ЃЉ+cЃЈH+ЃЉ=cЃЈOH-ЃЉ+ cЃЈHA-ЃЉЃЌдђcЃЈK+ЃЉ+cЃЈH+ЃЉ=cЃЈOH-ЃЉ+ cЃЈH2AЃЉЃЌЙЪAе§ШЗЃЛ
BЯюЁЂгЩЭМЯѓПЩжЊЃЌpH=4.2ЪБЃЌcЃЈH+ЃЉ=10-4.2mol/LЃЌcЃЈHA-ЃЉ=cЃЈA2-ЃЉЃЌK2ЃЈH2AЃЉ=![]() =cЃЈH+ЃЉ=10-4.2ЃЌЙЪBе§ШЗЃЛ
=cЃЈH+ЃЉ=10-4.2ЃЌЙЪBе§ШЗЃЛ
CЯюЁЂгЩЭМЯѓПЩжЊЃЌpH=2.7ЪБЃЌcЃЈH2AЃЉ=cЃЈA2-ЃЉЃЌгЩзнзјБъЪ§ОнПЩжЊcЃЈHA-ЃЉЃОcЃЈH2AЃЉ=cЃЈA2-ЃЉЃЌЙЪCе§ШЗЃЛ
DЯюЁЂгЩЭМЯѓПЩжЊЃЌKHAШмвКЯдЫсадЃЌЫЕУїШмвКжаHA-ЕчРыДѓгкЫЎНтЃЌдђcЃЈH+ЃЉЃОcЃЈOH-ЃЉЃЌcЃЈA2-ЃЉЃОcЃЈH2AЃЉЃЌЙЪDДэЮѓЁЃ
ЙЪбЁDЁЃ

ЁОЬтФПЁПNOxЪЧдьГЩДѓЦјЮлШОЕФжївЊЮяжЪЃЌгУЛЙдЗЈНЋЦфзЊЛЏЮЊЮоЮлШОЕФЮяжЪЃЌЖдгкЯћГ§ЛЗОГЮлШОгаживЊвтвхЁЃ
ЃЈ1ЃЉвбжЊЃК2C(s)+O2(g)![]() 2CO(g) ЁїH1= -221.0 kJ/mol
2CO(g) ЁїH1= -221.0 kJ/mol
N2(g)+O2(g)![]() 2NO (g) ЁїH2= +180.5 kJ/mol
2NO (g) ЁїH2= +180.5 kJ/mol
2NO(g)+2CO(g)![]() 2CO2(g)+N2(g) ЁїH3= -746.0 kJ/mol
2CO2(g)+N2(g) ЁїH3= -746.0 kJ/mol
ЛиД№ЯТСаЮЪЬтЃК
ЂйгУНЙЬПЛЙдNOЩњГЩЮоЮлШОЦјЬхЕФШШЛЏбЇЗНГЬЪНЮЊ_______ЁЃ
ЂкдквЛЖЈЮТЖШЯТЃЌЯђМзЁЂввЁЂБћШ§ИіКуШнУмБеШнЦїжаМгШывЛЖЈСПЕФNOКЭзуСПЕФНЙЬПЃЌЗДгІЙ§ГЬжаВтЕУИїШнЦїжаc(NO)ЃЈmol/LЃЉЫцЪБМфЃЈsЃЉЕФБфЛЏШчЯТБэЁЃ
вбжЊЃКШ§ИіШнЦїЕФЗДгІЮТЖШЗжБ№ЮЊTМз= 400ЁцЁЂTвв= 400ЁцЁЂTБћ= a Ёц
ЪБМф | 0 s | 10 s | 20 s | 30 s | 40 s |
Мз | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
вв | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
Бћ | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
МзШнЦїжаЃЌИУЗДгІЕФЦНКтГЃЪ§K=_______ЁЃБћШнЦїЕФЗДгІЮТЖШa _______400ЁцЃЈЬюЁАЁБЁЂЁА<ЁБЛђЁА=ЁБЃЉЃЌРэгЩЪЧ_______ЁЃ
ЃЈ2ЃЉгУNH3ДпЛЏЛЙдNOxЯћГ§ЕЊбѕЛЏЮяЕФЮлШОЁЃ
вбжЊЃК8NH3(g)+6NO2(g)![]() 7N2(g) +12H2O(l) ЁїHЃМ0ЁЃЯрЭЌЬѕМўЯТЃЌдк2 LУмБеШнЦїФкЃЌбЁгУВЛЭЌЕФДпЛЏМСНјааЗДгІЃЌВњЩњN2ЕФСПЫцЪБМфБфЛЏШчЭМЫљЪОЁЃ
7N2(g) +12H2O(l) ЁїHЃМ0ЁЃЯрЭЌЬѕМўЯТЃЌдк2 LУмБеШнЦїФкЃЌбЁгУВЛЭЌЕФДпЛЏМСНјааЗДгІЃЌВњЩњN2ЕФСПЫцЪБМфБфЛЏШчЭМЫљЪОЁЃ

ЂйдкДпЛЏМСAЕФзїгУЯТЃЌ0ЁЋ4 minЕФv(NH3) = _______ЁЃ
ЂкИУЗДгІЛюЛЏФмEa(A)ЁЂEa(B)ЁЂEa(C)гЩДѓЕНаЁЕФЫГађЪЧ_______ЃЌРэгЩЪЧ_______ЁЃ
ЂлЯТСаЫЕЗЈе§ШЗЕФЪЧ_______ЃЈЬюБъКХЃЉЁЃ
aЃЎЪЙгУДпЛЏМСAДяЦНКтЪБЃЌЁїHжЕИќДѓ
bЃЎЩ§ИпЮТЖШПЩЪЙШнЦїФкЦјЬхбеЩЋМгЩю
cЃЎЕЅЮЛЪБМфФкаЮГЩN-HМќгыO-HМќЕФЪ§ФПЯрЕШЪБЃЌЫЕУїЗДгІвбОДяЕНЦНКт
dЃЎШєдкКуШнОјШШЕФУмБеШнЦїжаЗДгІЃЌЕБЦНКтГЃЪ§ВЛБфЪБЃЌЫЕУїЗДгІвбОДяЕНЦНКт