��Ŀ����
(15��)
�Ķ��������ϣ��ݴ��������Ҫ��
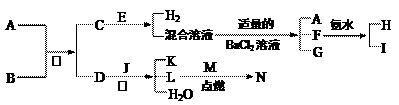 A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ
A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ
���У�A������ı���Ʒ������ɫ��Ӧ�ʻ�ɫ��BΪ��Ԫǿ�DΪ�⻯�E�ڵؿ��еĺ����ӵ���λ���䵥�ʴ����������쵼�ߺͺϽ�J����ͨ�ɵ�ص���Ҫ�ɷ֣�CΪ��ʽ�Σ�F��ˮ��Һ�������Ĺ������Ʒ�ӦҲ�ɵõ�I��G��һ�ֲ�����ϡ����İ�ɫ������M��L������ȼ�ղ����ػ�ɫ�̡�
��A�ľ�������Ϊ ��C�Ļ�ѧʽΪ ��
��M�Ļ�ѧʽΪ ����N��ˮ��Һ������ɫ����M��B��Ӧ�Ļ�ѧ����
ʽΪ�� ��
��D��Ũ��Һ��J��Ӧ�����ӷ���ʽΪ�� ��F��ˮ��Һ������Ĺ������Ʒ�Ӧ�����ӷ���ʽΪ�� ��
��H��Һ�и�����Ũ����С�����˳��Ϊ ��
�Ķ��������ϣ��ݴ��������Ҫ��
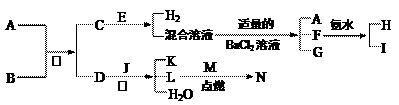 A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ
A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ���У�A������ı���Ʒ������ɫ��Ӧ�ʻ�ɫ��BΪ��Ԫǿ�DΪ�⻯�E�ڵؿ��еĺ����ӵ���λ���䵥�ʴ����������쵼�ߺͺϽ�J����ͨ�ɵ�ص���Ҫ�ɷ֣�CΪ��ʽ�Σ�F��ˮ��Һ�������Ĺ������Ʒ�ӦҲ�ɵõ�I��G��һ�ֲ�����ϡ����İ�ɫ������M��L������ȼ�ղ����ػ�ɫ�̡�
��A�ľ�������Ϊ ��C�Ļ�ѧʽΪ ��
��M�Ļ�ѧʽΪ ����N��ˮ��Һ������ɫ����M��B��Ӧ�Ļ�ѧ����
ʽΪ�� ��
��D��Ũ��Һ��J��Ӧ�����ӷ���ʽΪ�� ��F��ˮ��Һ������Ĺ������Ʒ�Ӧ�����ӷ���ʽΪ�� ��
��H��Һ�и�����Ũ����С�����˳��Ϊ ��
(15��)
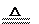 (1)���Ӿ���(2��) NaHSO4(2��)
(1)���Ӿ���(2��) NaHSO4(2��)
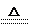 (2)Fe(��Cu) (2��) Cu+2H2SO4(Ũ) CuSO4+SO2��+H2O(2��)
(2)Fe(��Cu) (2��) Cu+2H2SO4(Ũ) CuSO4+SO2��+H2O(2��)
(3)MnO2+4H��+2Cl�� Mn2��+Cl2��+2H2O(2��)
(4)c(OH��)<c(H��)<c(NH4��)<c(Cl��) (3��)
 (1)���Ӿ���(2��) NaHSO4(2��)
(1)���Ӿ���(2��) NaHSO4(2��) (2)Fe(��Cu) (2��) Cu+2H2SO4(Ũ) CuSO4+SO2��+H2O(2��)
(2)Fe(��Cu) (2��) Cu+2H2SO4(Ũ) CuSO4+SO2��+H2O(2��)(3)MnO2+4H��+2Cl�� Mn2��+Cl2��+2H2O(2��)
(4)c(OH��)<c(H��)<c(NH4��)<c(Cl��) (3��)
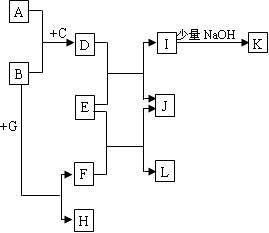
�����ǵ��͵����ƶϣ�ֻҪ�Ƴ���������ܺý���ˣ�A NaCl��B H2SO4��C NaHSO4��D HCl��J MnO2;K MnCl2;L Cl2;M Cu��Fe;N CuCl2��FeCl3;F AlCl3;G BaSO4; H NH4Cl;I Al(OH)3��������ͺ��������

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ

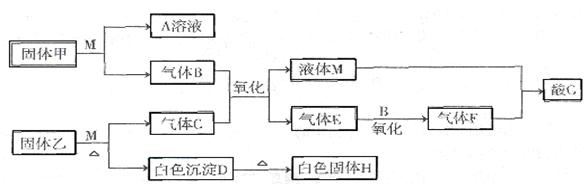
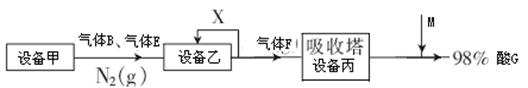
 Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��
Һ����Һ������Ũ���ɴ�С��˳��Ϊ____________ ___��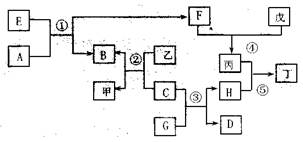
 2I��g����
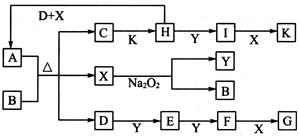
2I��g����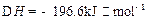 ��������4 mol H��2 mol Y�ų�345 kJ������ʱ��H��ת������ӽ���__________������ĸ����
��������4 mol H��2 mol Y�ų�345 kJ������ʱ��H��ת������ӽ���__________������ĸ����