��Ŀ����
����Ŀ��ij��Һ�к��е����ӿ�����K+��Ba2+��Al3+��Mg2+��![]() ��
��![]() ��
��![]() ��Cl���еļ��֣��ֽ�������ʵ�飺
��Cl���еļ��֣��ֽ�������ʵ�飺
��ȡ����ԭ��Һ���μ�����������Һ�Ĺ������������ɣ�
����ȡ����ԭ��Һ����μ���5 mL 0.2 mol��L-1���ᣬ��ʼ���������������࣬���������������������壬��������������ʧ��
����������������ʧ�����Һ�У��ټ�����������������Һ�õ�����0.43 g��
����˵������ȷ����(����)��
A.����Һ��һ������Ba2+��Al3+��Mg2+��![]() ��Cl��
��Cl��
B.����Һ��һ������K+��![]() ��
��![]() ��Cl��
��Cl��
C.����Һ���Ƿ���K+������ɫ��Ӧ(����ɫ�ܲ���Ƭ�۲�)�����ж�
D.����Һ�п��ܺ���Cl��
���𰸡�B
��������
ij��Һ�к��е����ӿ�����K+��Ba2+��Al3+��Mg2+��![]() ��
��![]() ��
��![]() ��Cl���еļ��֣���ȡ����ԭ��Һ���μ�����������Һ�Ĺ������������ɣ�˵����Al3+��Mg2+������ȡ����ԭ��Һ����μ���5 mL 0.2 mol��L1���ἴ���ʵ���Ϊ0.001mol����ʼ���������������࣬���������������������壬��������������ʧ��˵������
��Cl���еļ��֣���ȡ����ԭ��Һ���μ�����������Һ�Ĺ������������ɣ�˵����Al3+��Mg2+������ȡ����ԭ��Һ����μ���5 mL 0.2 mol��L1���ἴ���ʵ���Ϊ0.001mol����ʼ���������������࣬���������������������壬��������������ʧ��˵������![]() ��
��![]() ����
����![]() ������̼����ͱ����Ӳ����棬�õ���Ba2+������������������ʧ�����Һ�У��ټ�����������������Һ�õ�����0.43 g��AgCl���ʵ���Ϊ0.003mol����˵��ԭ��Һ�к���Cl����
������̼����ͱ����Ӳ����棬�õ���Ba2+������������������ʧ�����Һ�У��ټ�����������������Һ�õ�����0.43 g��AgCl���ʵ���Ϊ0.003mol����˵��ԭ��Һ�к���Cl����
A. ����ǰ������õ�����Һ��һ������Ba2+��Al3+��Mg2+��![]() ����A����
����A����
B. ����ǰ������õ�����Һ��һ������![]() ��
��![]() ��Cl����������Һ�ʵ����ԣ����һ������K+����B��ȷ��
��Cl����������Һ�ʵ����ԣ����һ������K+����B��ȷ��
C. ������Һ����غ�õ���Һ����K+����������ɫ��Ӧʵ�飬��C����
D. ����ǰ�������Ϣ�õ�����Һ��һ������Cl������D����
������������ΪB��

����Ŀ����1����֪��H2(g)��![]() O2(g)=H2O(g) ��H����241.8kJ��mol��1
O2(g)=H2O(g) ��H����241.8kJ��mol��1
��CH4 (g)��![]() O2(g)=CO (g)��2H2O(g) ��H����564.3kJ��mol��1
O2(g)=CO (g)��2H2O(g) ��H����564.3kJ��mol��1
��CH4(g)��H2O(g)��Ӧ��ȡCO(g)��H2(g)���Ȼ�ѧ����ʽ��___��
��2�����ݼ������ݼ���CH4(g)+4F2(g)=CF4(g)+4HF(g)�ķ�Ӧ�� ��H��___kJ��mol��1��
��ѧ�� | C��H | C��F | H��F | F��F |
����/(kJ��mol-1) | 414 | 489 | 565 | 155 |
��3�������Ǻϳɰ���Ӧ����Ҫԭ�ϡ��ֽ�lmolN2��3molH2Ͷ��1L���ܱ���������һ�������£��������·�Ӧģ��ϳɰ��Ĺ�ҵ��������N2(g)+3H2(g)![]() 2NH3(g) ��H��0�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��NH3�����������(NH3)�仯������ͼ��ʾ��
2NH3(g) ��H��0�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��NH3�����������(NH3)�仯������ͼ��ʾ��
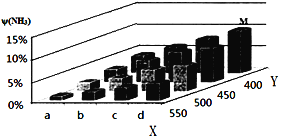
�ش��������⣺
��ƽ��ʱ��M��NH3���������Ϊ10%����N2�����ʵ���Ϊ___(������λ��Ч����)��
��X����a�����ֵ��b��__(������������С��)��ͼ�У�Y���ʾ__(�����¶�������ѹǿ��)���жϵ�������__��