��Ŀ����
17��һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���1molN2���ұ߳���CO��CO2�Ļ�����干8gʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ���ǣ�������
| A�� | �ұ�CO��CO2������֮��Ϊ3��1 | |
| B�� | �Ҳ�CO������Ϊ2.75g | |
| C�� | �Ҳ������ܶ�����ͬ�����������ܶȵ�16�� | |
| D�� | ���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/6���������¶Ȳ��䣬��ǰ�����γ�����������ڵ�ѹǿ֮��Ϊ24��25 |
���� �������������¶ȡ�ѹǿ��ͬ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��������֮��Ϊ4��1���������������ʵ���֮��Ϊ4��1�������Ҳ��������ʵ���=$\frac{1mol}{4}$=0.25mol��CO�Ͷ�����̼����Ϊ8g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ��0.25-x��mol��28xg+44��0.25-x��g=8g��x=$\frac{3}{16}$mol����CO�����ʵ���Ϊ$\frac{3}{16}$mol��������̼���ʵ���Ϊ$\frac{1}{16}$mol��
A����������ʵ���֮�ȵ����������֮�ȣ�
B������m=nM����CO������
C����ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ�
D�����ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/6���������ҿռ����֮��Ϊ5��1�����������̼��CO���ʵ���Ϊ$\frac{1mol}{5}��1$=0.2mol����ͬ��������������ʵ���֮�ȵ�����ѹǿ֮�ȣ�
��� �⣺�������������¶ȡ�ѹǿ��ͬ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��������֮��Ϊ4��1���������������ʵ���֮��Ϊ4��1�������Ҳ��������ʵ���=$\frac{1mol}{4}$=0.25mol��CO�Ͷ�����̼����Ϊ8g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ��0.25-x��mol��28xg+44��0.25-x��g=8g��x=$\frac{3}{16}$mol����CO�����ʵ���Ϊ$\frac{3}{16}$mol��������̼���ʵ���Ϊ$\frac{1}{16}$mol��
A����������ʵ���֮�ȵ����������֮�ȣ������ұ�CO��CO2������֮��Ϊ$\frac{3}{16}$mol��$\frac{1}{16}$mol=3��1����A��ȷ��
B��m��CO��=nM=$\frac{3}{16}mol��28g/mol$=5.25g����B����
C����ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ��ұ�����ƽ��Ħ������=$\frac{8g}{0.25mol}$=32g/mol��������Ħ��������ȣ����Ի�������������ܶ�֮��Ϊ1��1����C����
D�����ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/6���������ҿռ����֮��Ϊ5��1�����������̼��CO���ʵ���Ϊ$\frac{1mol}{5}��1$=0.2mol����ͬ��������������ʵ���֮�ȵ�����ѹǿ֮�ȣ�������ѹǿ֮��Ϊ0.25mol��0.2mol=5��4����D����
��ѡA��
���� ���⿼�����ʵ����йؼ��㣬Ϊ��Ƶ���㣬���ؿ���ѧ������������������ȷ��������������Ϊ���º�ѹ�����ǽⱾ��ؼ����״�ѡ����C��

| A�� | ������ɫ������һ�����ɫ������ɫ��˵����Һ����Cl2���� | |
| B�� | ��Һ�ʻ���ɫ�����д̼�����ζ��˵����Cl2���Ӵ��� | |
| C�� | ����AgNO3��Һ������ɫ������˵����Cl-���� | |
| D�� | ����NaOH��Һ����ˮ����ɫ��ʧ��˵����HClO���Ӵ��� |
| A�� | K+��MnO4-��S2-��SO42- | B�� | K+��OH-��Cl-��CO32- | ||
| C�� | Ca2+��CH3COO-��Al3+��SO42- | D�� | Na+��Ba2+��Cl-��NO3- |
| �ı����� | ƽ���ƶ����� | n��H+�� | c��H+�� | c��CH3COO-�� |
| ���������Ĵ����ƹ��� | ||||
| ����þ�� | ||||
| ����һ����ˮϡ�� | ||||
| ����һ����ˮϡ�� | ||||
| ����Ũ���� | ||||
| ����NaOH ���� |
| A�� | NaHSO4��Һ | B�� | NH4NO3��Һ | C�� | KAl��SO4��2 ��Һ | D�� | NaCl��Һ |
| ������ | ����� | ǿ����� | ������� | �ǵ���� | |
| A | ��ˮ | ��ˮ | ���� | ���� | �� �� |
| B | ������ | ���� | ���ᱵ | �� ���� | �������� |
| C | ���� | ��� | ���Լ� | ������ | ̼��� |
| D | �Ȼ��� | ����������Һ | �Ȼ��� | ���� | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
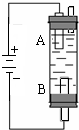 ijͬѧ�����һ�ֵ�ⷨ��ȡFe��OH��2��ʵ��װ�ã���ͼ�����������ڵĵ��ҺΪNaCl��Һ��ͨ�����Һ�в�����ɫ�������ҽϳ�ʱ�䲻��ɫ���ش��������⣺
ijͬѧ�����һ�ֵ�ⷨ��ȡFe��OH��2��ʵ��װ�ã���ͼ�����������ڵĵ��ҺΪNaCl��Һ��ͨ�����Һ�в�����ɫ�������ҽϳ�ʱ�䲻��ɫ���ش��������⣺