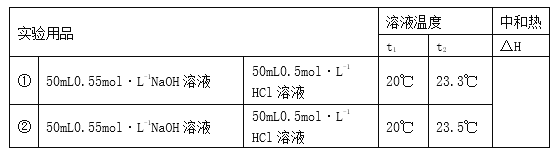
��Ŀ����
����Ŀ����Ҫ����գ�
��1��0.2molO3��0.3molO2������___________��ѡ���������������������������ж�������������֮��Ϊ__________������ԭ����֮��Ϊ___________������ͬ�����µ����֮��Ϊ_____________��
��2��73gHCl�����к���_____________�����ӣ���״����ռ�е����Ϊ_____________L��
��3����ͬ���ʵ���Ũ�ȵ�KCl��MgCl2��AlCl3������Һ���ֱ���AgNO3��Һ��Ӧ�������ɵ�AgCl�ij���������֮��Ϊ3��2��1ʱ��������Һ�������Ϊ__________��
��4����״����VL�Ȼ��������ܽ���1Lˮ�У�������Һ���ܶ�Ϊ��gmL����Һ�����ʵ���������Ϊ�������ʵ���Ũ��ΪcmolL-1������=__________����дһ�ֱ���ʽ����
���𰸡��������1����ȡ������2��2��3�������3��1��1�������4��2��3�������5��2NA�������6��44.8�������7��9��3��1�������8��![]()
��������
��1��0.2molO3��0.3molO2��������Ϊ9.6g������ȣ�������֮�ȵ������ǵ����ʵ���֮�ȣ�Ϊ2:3��0.2molO3���е���ԭ�ӵ����ʵ��� 0.6mol��0.3molO2���е���ԭ�ӵ����ʵ��� 0.6mol����������ԭ����֮��Ϊ1:1������ͬ�����µ����֮�ȵ������ǵ����ʵ���֮�ȣ�Ϊ2:3��
��2��73gHCl���ʵ���=![]() =2mol��HCl������Ŀ=2mol��NAmol-1=2NA������������=2mol��22.4L/mol=44.8L��
=2mol��HCl������Ŀ=2mol��NAmol-1=2NA������������=2mol��22.4L/mol=44.8L��
��3����������ӦAg++Cl-=AgCl�������ɵ�AgCl����������֮��Ϊ3��2��1����KCl��CuCl2��AlCl3���е����������ʵ���֮��Ϊ3��2��1����n��KCl����n��CuCl2����n��AlCl3��=3��![]() ��
��![]() =9��3��1��Ũ����ͬ���֮�ȵ��ڸ����ʵ����ʵ���֮��Ϊ9��3��1��
=9��3��1��Ũ����ͬ���֮�ȵ��ڸ����ʵ����ʵ���֮��Ϊ9��3��1��
��4������c=![]() ��ʽ��֪��=
��ʽ��֪��=![]() ��
��