��Ŀ����
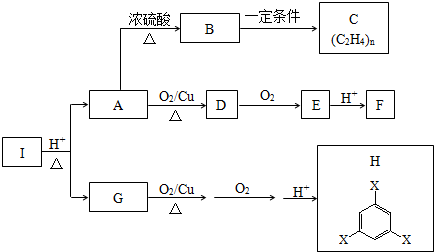
16��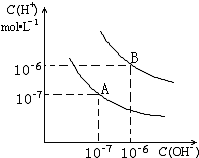 ��֪ˮ��25���95��ʱ������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ������ƽ��������ͼ��ʾ����1����25��ʱˮ�ĵ���ƽ������ӦΪA���A����B��������˵������ˮ�ĵ���ʱ���ȹ��̣����ȴٽ����룬�¶ȵͣ�ˮ�����ӻ�С
��2��25��ʱ����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ�� pH=7����NaOH��Һ��H2SO4��Һ�������Ϊ10��1
��3��95��ʱ����100���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��pH1+pH2=14����a+b=14
��4������B��Ӧ�¶��£�pH=2��ijHA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���������ԭ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
���� ��1��������������Ũ�ȣ�����������������Ũ�ȣ�ˮ�����ӻ�����Kw=c��H+����c��OH-�������A���ߵ�Kwֵ���ˮ�ĵ���ʱ���ȱȽ��ж�25��ʱˮ�ĵ���ƽ�����ߣ�
��2��������Һ��pH�������Һ�������ӡ�����������Ũ�ȣ�����ʽ���������������Һ��������Һ�������
��3���ᡢ���ǿ����ʣ���Һ������˵�������Ӻ����������ӵ����ʵ�����ȣ����ˮ�����ӻ�����ȷ��ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��
��4����������B��Ӧ�¶���pH=5��˵����Һ��ʾ���ԣ���Ӧ�������ӹ���������
��� �⣺��1������A������Kw=c��H+����c��OH-��=10-7��10-7=10-14������B������c��H+��=c��OH-��=10-6 mol/L��Kw=c��H+��•c��OH-��=10-12 ��ˮ�ĵ���ʱ���ȹ��̣����ȴٽ����룬����A���ߴ���25��ʱˮ�ĵ���ƽ�����ߣ�
�ʴ�Ϊ��A��ˮ�ĵ���ʱ���ȹ��̣����ȴٽ����룬�¶ȵͣ�ˮ�����ӻ�С��
��2��25��ʱ���û����Һ��pH=7����Һ�����Լ����ǡ���кͣ���n��OH-��=n��H+������V��NaOH��•10-5 mol•L-1=V��H2SO4��•10-4 mol•L-1����V��NaOH����V��H2SO4��=10��1���ʴ�Ϊ��10��1��
��3��Ҫע�����95��Cʱ��ˮ�����ӻ�Ϊ10-12�����ᡢ��Ũ�����ʱpH���ᣩ+pH���=12����ǿ���OH-Ũ����ǿ��H+Ũ�ȵ�100��������pH���ᣩ+pH���=14����pH1+pH2=14����a+b=14���ʴ�Ϊ��pH1+pH2=14����a+b=14��
��4��������B��Ӧ�¶��£���pH���ᣩ+pH���=12���ɵ��������Һ��c��H+��=c��OH-��������ǿ������Һ�������Ϻ���Һ�����ԣ��ֻ����Һ��pH=5�����������Ϻ���Һ�����ԣ�˵��H+��OH-��ȫ��Ӧ�������µ�H+�������������������HA�����ᣬ
�ʴ�Ϊ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
���� ���⿼����ˮ�ĵ��롢ˮ�����ӻ�����ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ�����ؼ����ڸ�����¶ȶ�ˮ����ƽ�⡢ˮ�����ӻ�����ҺpH��Ӱ�죮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | CH3CH2CHO��HCHO | B�� |  OH�� OH�� CH2OH CH2OH | ||
| C�� | ������HCOOCH3 | D�� | CH3CH2Cl��CH3CH2CH2Br |
| A�� | 612C��613C | B�� | O2��O3 | C�� | H2O��H2O2 | D�� | CO��CO2 |
| A�� | Ϊ�˼�����Ӧ���ʿ��ñ���ʳ��ˮ����ˮ���з�Ӧ | |
| B�� | �÷�ӦΪ���ȷ�Ӧ | |
| C�� | ������Ȳ�ķ�ӦΪCaC2+H2O��CaO+CH��CH�� | |
| D�� | Ϊ�˳�ȥ��Ȳ�����е����ʿ�����CuSO4��Һϴ�� |
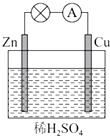 ��ͼ��ʾ��Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼���£���Ƭ�������������ǣ�������
��ͼ��ʾ��Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼���£���Ƭ�������������ǣ�������| A�� | CuΪ������ZnΪ���� | B�� | Cu���������ݲ�����������ԭ��Ӧ | ||
| C�� | SO42-��Cu���ƶ� | D�� | ���ӵ������ǣ�Cu-��Zn |
| A�� | 250mL��23.4g | B�� | 250mL��29.3g | C�� | 500mL��29.3g | D�� | 500 mL��58.5g |
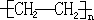 ����Ӧ�����ǼӾ۷�Ӧ��
����Ӧ�����ǼӾ۷�Ӧ�� _��
_��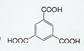 ��
��