��Ŀ����
Ϊ�ⶨij��Ѫ����Ʒ����Ҫ�ɷ���������������(FeSO4��7H2O)������Ԫ�صĺ�����ij��ѧ��ȤС�����������ʵ�鷽��:
����һ:������KMnO4��Һ�ζ��ⶨ��Ԫ�صĺ���
��д���ζ���Ӧ�����ӷ���ʽ ��
(2)���еζ���ʽ�У���������� (�г�������ȥ)(����ĸ��š�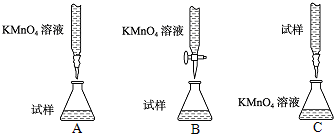
(3)ʵ��ǰ������Ҫȷ����һ�����ʵ���Ũ�ȵ�����KMnO4��Һ250mL,����ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����_ (����������)��
(4)��ijͬѧȡ5Ƭ��Ѫ����Ʒ���100 mL��Һ��ȡ20. 00 mL��������Ũ��Ϊc1mol��L-1��KMnO4��Һ�ζ�����ȥV1 mL����ÿƬ��Ѫ������Ԫ�ص�����_ g(�ô���ʽ��ʾ)��
������:��FeSO4ת��ΪFe2O3���ⶨ�����仯��������������:
(5)���������H2O2��Ŀ����_ _��
(6)�������һϵ�в���������:���ˡ�ϴ�ӡ�_ ����ȴ��������
(7)����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ_ g(�ú�a�Ĵ���ʽ��ʾ)��
(8)��ijͬѧ�����ԭ��ص���ʽʵ��Fe2+��Fe3+��ת��������ͨ��O2���������ҺΪϡ���ᣬ��д�������ĵ缫��Ӧʽ ��
��15�֣�1��5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O ��2�֣� ��2��B ��1�֣�
��3��50mL����ƿ��2�֣��������0�֣� ��4��0.28c1v1��2�֣�
��5����Fe2��������Fe3����2�֣� ��6������ ��1�֣� ��7��0.07a ��2�֣�
��8��O2��4e����4H����2H2O��2�֣�
���������������1�����Ը�����ؾ���ǿ�����ԣ���Fe2+����ΪFe3+��MnO4������ԭΪMn2+��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
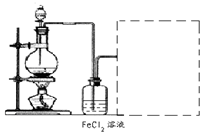
��2�����Ը�����ؾ���ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧʢ������ʽ�ζ����ڣ�����������Һ�����ԣ�Ӧʢ������ʽ�ζ����ڣ�װ��A��C�о��Ǽ�ʽ�ζ��ܣ�����B����ʡ�
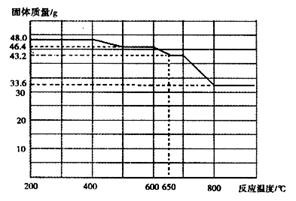
��3����ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ��ҩ�ס����������ձ�����ͷ�ιܡ�250mL����ƿ��Զ�̻�ȱ��250mL����ƿ��
��4�����ĸ�����ص����ʵ�����0.001c1v1mol�����ݷ���ʽ��֪���������ӵ����ʵ�����0.005c1v1mol����5Ƭ��Ѫ����Ʒ���������ӵ����ʵ�����0.005c1v1mol��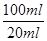 ��0.025c1v1mol������ÿƬ��Ѫ����Ʒ���������ӵ����ʵ�����0.005c1v1mol�����ÿƬ��Ѫ������Ԫ�ص�������0.005c1v1mol��56g/mol��0.28c1v1g��
��0.025c1v1mol������ÿƬ��Ѫ����Ʒ���������ӵ����ʵ�����0.005c1v1mol�����ÿƬ��Ѫ������Ԫ�ص�������0.005c1v1mol��56g/mol��0.28c1v1g��
��5��Ҫ�����������������������ӣ�����Ҫ������������˫��ˮ�dz��õ���ɫ�����������˫��ˮ�������ǽ�Fe2��������Fe3����
��6���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ������������Ȼ���������ɵ�����������ȴ�������������������
��7��������ԭ���غ��֪��ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ������������������ÿƬ��Ѫ������Ԫ�ص�����Ϊ ��0.07ag��
��0.07ag��
��8��ԭ����������õ����ӣ�������ԭ��Ӧ������������ͨ�룬����Һ�����ԣ����������缫��ӦʽΪO2��4e����4H����2H2O��
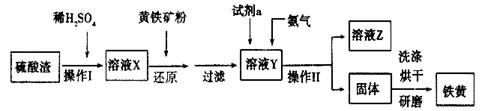
���㣺�������ʷ������ᴿ��Ԫ�ػ��������ʡ�������ԭ��Ӧ�ζ�������ѡ��ѧ�����Լ�ʵ�鷽����������۵�

 ��������������������ϵ�д�
��������������������ϵ�д���������������Ԫ�أ�����ȱ����ƶѪ�ij��������Ƿ��ò���ҩ��������ơ�(��Ҫ�ɷ֣��������������ʰ���ɫ)���г���һ�ֳ����IJ���ҩ���ҩƷ������ˮ�������������е�θ�ᡣ
ijͬѧΪ�˼�⡰�����ơ�ҩƬ��Fe2���Ĵ��ڣ���Ʋ�����������ʵ�飺
��1���Լ�1�� ������������ˮ����Һ�з��������ӷ�Ӧ����ʽ�ǣ� �� ��
��2������KSCN��Һ����δ��������ˮ������£���Һ��Ҳ�����˺�ɫ������ܵ�ԭ���� ��
��3����ʵ���з��ַ���һ��ʱ�䣬��Һ����ɫ������ȥ��Ϊ�˽�һ��̽����Һ��ɫ��ԭ�ס��ҡ�����λͬѧ���Ƚ����˲��룺
| ��� | �� �� |
| �� | ��Һ�еģ�3��Fe�ֱ���ԭΪ��2��Fe |
| �� | ��Һ�е�SCN������������ˮ���� |
| �� | ���Ƶ���ˮ����Ư���ԣ�������ҺƯ�� |
| ��� | ʵ����� | Ԥ������ͽ��� |
| �� | | |
| | | |
| | | |
�Ȼ�������Һ�еμ����軯����Һ���ٵμ�˫��ˮ����������Ѫ��ɫ�����ɫ����ȥ�ʻ�ɫ���������ݲ�������Ը�ʵ������ijʵ��С���ͬѧ������̽��
һ���������
����A��������H2O2�ֽ������O2
����B��������KSCN������ΪN2��SO2��CO2��
����C����ɫ��ȥ��ԭ����KSCN������������������
����ʵ��̽��
̽��1
| ʵ����� | ʵ������ | ���� |
| ��1mL 0.1mol/L��FeCl2��Һ�м�2��KSCN��Һ | ��Һ����� | Fe2+��SCN-����� |
| ����ٵ���Һ�м�3%��H2O21�β��� | ��������Ѫ��ɫ ����ɫ | ����H2O2����Һ�� ������ (���ӷ���) |
| ����ڵ���Һ�м�����H2O2��Һ | ��Һ�г��ִ������� Ѫ��ɫ��ȥ | |
| ���ô����ǵ�ľ��������е����� | ľ����ȼ | ����� ���� |
̽��2
| ʵ����� | ʵ������ | ���� |
| ��ȡ2ml KSCN��Һ�����м��뼸��BaCl2��Һ��ϡ���� | ���������� | |
| ��������õ���Һ�еμ�3%��H2O2 | ��Һ�г��ְ�ɫ���������������� | ��ɫ����ΪBaSO4 |
| �۽�6%��H2O2��Һ����KSCN�����У����ɵ���������ͨ��Ʒ����Һ������KMnO4��Һ�ͳ����ʯ��ˮ | | KSCN��H2O2����������SO2��CO2���� |
(1)̽��1�У�H2O2�ֽ��ٶȺܿ��ԭ��
(2)̽��1�У�˵����ԭ��Fe2+ SCN-
(3)̽��2�У�����KMnO4��Һ��������
��֤��������CO2��������
(4)��SCN -��H2O2�����õ�N2��SO2��CO2��SO42-����SO2��SO42-�����ʵ�����Ϊ1:1����д���÷�Ӧ�����ӷ���ʽ


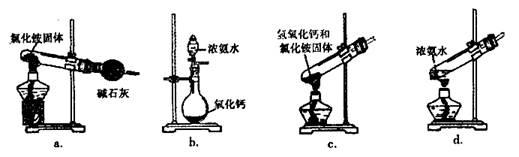
 �ķ����� ��
�ķ����� ��