��Ŀ����
������ʵĵ���ƽ�⡢�����ˮ��ƽ�����������ܽ�ƽ������ڻ�ѧƽ�⡣����Ҫ��ش����}��
(1)����������������ˮ�����侻ˮ��ԭ����_____________(�����ӷ���ʽ��ʾ)��
(2)�����£�ȡ0.2 mol/L HCl��Һ��0.2mol/L MOH��Һ�������ϣ���û�Ϻ���Һ��pH=5��д��MOH�ĵ��뷽��ʽ��__________________��
 (3)0.1mol/L��NaHA��Һ�������Һ�Լ��ԡ���
(3)0.1mol/L��NaHA��Һ�������Һ�Լ��ԡ���
�� ����Һ�� c(H2A)_______________c(A2-)(�>������<����=��)
�� ���������жϵ�������_____________(�����ֽ���)��
(4)��Cr2O72-�ķ�ˮ���Խϴ�ij������ˮ�к�5.0��10-3 mol/L��Cr2O72-��Ϊ��ʹ��ˮ���ŷŴ�꣬�������´�����

�̷�ΪFeSO4• 7H2O����Ӧ(I)��Cr2O72-��FeSO4�����ʵ���֮��Ϊ___________��
��������������ķ�ˮ��c(Cr3+)=6.0��10-7mol/L��������ķ�ˮ�� pH=___________��
{Ksp[Cr(OH)3]=6.0��10-31}
 �Ķ��쳵ϵ�д�
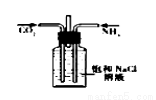
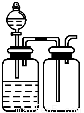
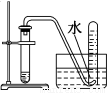
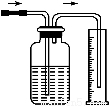
�Ķ��쳵ϵ�д�����ѡ�õ�ʵ��������ʵ��װ�÷���ʵ��Ҫ���Ұ�ȫ����( )
A | B | C | D |
��NaCl��Һ��ͨNH3����ͨCO2�Ʊ�NaHCO3 | ʵ�����Ʊ��������� | ʵ�����Ʊ�Cl2 | ����O2��� |
|
|
|
|



 ʱ��1 mol•L-1�Ĵ�����Һ����ƽ�ⳣ��Ϊ1��0��10 -8����ƽ��ʱ����Һ��������Ũ���� ��
ʱ��1 mol•L-1�Ĵ�����Һ����ƽ�ⳣ��Ϊ1��0��10 -8����ƽ��ʱ����Һ��������Ũ���� ��