��Ŀ����
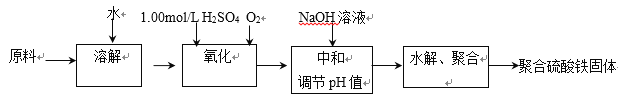
����Ŀ���ۺ���������һ������Ч�����߷������������������������弰����Ϊԭ�ϴ���������������������ˮ�⡢�ۺϳɲ�Ʒ��ʵ����ģ�������������£�
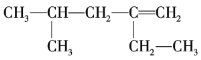
(1)����ԭ������2.50 mol/L��FeSO4��Һʱ�õ��Ķ��������У�____________________
��д�����������е����ӷ���ʽ��_________________________________________
(2)�ۺϿ���ʵ��Ͷ��������������������ʵ���֮��Ϊ![]() ������ѡ� ��������������ֵ�Զ࣬�����ܹ����ԭ����____________________________________________________________________��
������ѡ� ��������������ֵ�Զ࣬�����ܹ����ԭ����____________________________________________________________________��
(3)��������Һˮ����Եõ�һϵ�о��о�ˮ���õļ�ʽ������(x Fe2O3��y SO3��z H2O)���ֲ����������ⶨx��y��z��ֵ��
�ٲⶨʱ������Լ�____________��
(a) NaOH (b) Ba(OH)2 (c) BaCl2 (d) FeSO4
����Ҫ�ⶨ____________��____________������(��д������Ļ�ѧʽ)��
(4)ѡ���ⶨ����������Ļ�������____________(�������Ⱥ�˳���г�)��
a.���ˡ�ϴ��
b.�������ᾧ
c.��ȡ����Һ
d.��ȴ������
e.��ɻ�����
���𰸡�������ƽ������ƿ 4Fe2+ + O2 + 4H+![]() 4Fe3+ + 2H2O �����Թ�����Ϊ�˷�ֹFe3+ˮ����������������������ʱ�������к���Ҫ�ļ������������˷� ac Fe2O3 BaSO4 aed
4Fe3+ + 2H2O �����Թ�����Ϊ�˷�ֹFe3+ˮ����������������������ʱ�������к���Ҫ�ļ������������˷� ac Fe2O3 BaSO4 aed
��������
(1)������һ�����ʵ���Ũ�ȵ���ҺҪ�������ʵ�������������ƿ���ݣ�
���������������������±�������������������
(2)������࣬�ں������pHʱҪ���ĵ��������Ƶ����Ͷ࣬����˷ѣ�
(3)�ٲ����������ⶨ��ʽ������(x Fe2O3y SO3z H2O)��x��y��z��ֵʱ�����Խ���Ʒ��������������Һ�����ݵõ��Ĺ���������������ȷ��x��ֵ�����ˣ���������Һ�м��Ȼ��������ݲ��������ᱵ������������ȷ��y��ֵ��������Ʒ�����������Fe2O3�ͼ���õ�SO3��������ȷ��z��ֵ��
�ڸ��ݢٵķ�����֪��Ҫ�ⶨFe2O3��BaSO4��������
(4)�ⶨ�����н���Ʒ��������������Һ���������ˡ�ϴ�ӡ���ɻ����ա���ȴ������������������������������Һ�м��Ȼ������������ˡ�ϴ�ӡ���ɻ����ա���ȴ�����������ᱵ���������ݴ˴��⡣
(1)������һ�����ʵ���Ũ�ȵ���ҺҪ�������ʵ�������Ҫ�õ�����ƽ��������ƿ���ݣ��ʴ�Ϊ��������ƽ������ƿ��
���������������������±���������������������Ӧ�����ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O���ʴ�Ϊ��4Fe2++O2+4H+=4Fe3++2H2O��
(2)�����Թ�����Ϊ�˷�ֹFe3+ˮ����������������������ʱ�������к���Ҫ�ļ��������������˷ѣ����Լ�������������ֵ�Զ࣬�����ܹ��࣬�ʴ�Ϊ�������Թ�����Ϊ�˷�ֹFe3+ˮ����������������������ʱ�������к���Ҫ�ļ��������������˷ѣ�
(3)�ٲ����������ⶨ��ʽ������(x Fe2O3y SO3z H2O)��x��y��z��ֵʱ�����Խ���Ʒ��������������Һ�����ݵõ��Ĺ���������������ȷ��x��ֵ�����ˣ���������Һ�м��Ȼ��������ݲ��������ᱵ������������ȷ��y��ֵ��������Ʒ�����������Fe2O3�ͼ���õ�SO3��������ȷ��z��ֵ����ѡac��
�ڸ��ݢٵķ�����֪��Ҫ�ⶨFe2O3��BaSO4���������ʴ�Ϊ��Fe2O3��BaSO4��
(4)�ⶨ�����н���Ʒ��������������Һ���������ˡ�ϴ�ӡ���ɻ����ա���ȴ������������������������������Һ�м��Ȼ������������ˡ�ϴ�ӡ���ɻ����ա���ȴ�����������ᱵ����������ѡaed��