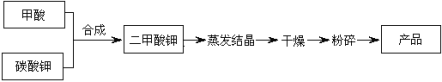
��Ŀ����
����Ŀ�����û�ѧ��Ӧԭ����������������⣺
��1��������ˮ��Һ�д��ڵ���ƽ�⣬����0.1 mol��L��1����ĵ���ƽ�ⳣ�����±���
���� | ����ƽ�ⳣ��(25 ��) |
HClO | K��2.98��10��8 |
H2CO3 | K1��4.3��10��7 K2��5.6��10��11 |
H2SO3 | K1��1.54��10��2 K2��1.02��10��7 |
���������Ũ��һ��ʱ�������¶ȣ�Kֵ________(�������������С������������)��
���������ӷ���ʽ���й�˵���������____________��
a��������CO2ͨ�����������Һ�У�2ClO����H2O��CO2===2HClO��CO32-
b��������SO2ͨ��̼������Һ�У�SO2��H2O��2CO32-===2HCO3-��SO32-
c����ͬ�¶�ʱ�������ʵ���Ũ�ȵ���������������NaOH��Һ��ȫ�к�����NaOH�����ΪV(H2CO3)��V(H2SO3)��V(HClO)
d����ͬ�¶�ʱ����pH��������Һ�����ʵ���Ũ�ȹ�ϵ��c(Na2CO3)��c(NaClO)��c(Na2SO3)
��������(H2SeO3)Ҳ��һ�ֶ�Ԫ���ᣬ�н�ǿ�������ԡ�����������Һ�в���ͨ��SO2��������ɫ���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��___ ��
��2����ҵ��ˮ�г�����һ������Cr2O72-��CrO42�������Ƕ����༰��̬ϵͳ������ܴ���������д������ŷš�
���ڷ�ˮ�д���ƽ�⣺2CrO42�� (��ɫ)��2H��![]() Cr2O72�� (��ɫ)��H2O�����ı�����ʹ����ƽ�����淴Ӧ�����ƶ���������˵����ȷ����_______��
Cr2O72�� (��ɫ)��H2O�����ı�����ʹ����ƽ�����淴Ӧ�����ƶ���������˵����ȷ����_______��
a��ƽ�ⳣ��Kֵ���Բ��ı�
b���ﵽ��ƽ��CrO42�����������ʵ���Cr2O72������������
c���ﵽ��ƽ�����ҺpHһ������
d���ٴ�ƽ��ǰ�淴Ӧ����һ����������Ӧ����
��Cr2O72����CrO42���������ɵ�Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s) ![]() Cr3��(aq)��3OH��(aq)��������Cr(OH)3��Ksp=10-32����c(Cr3��)����10��3 mol��L��1����Һ��pH����4ʱ��________(������������û����)����������
Cr3��(aq)��3OH��(aq)��������Cr(OH)3��Ksp=10-32����c(Cr3��)����10��3 mol��L��1����Һ��pH����4ʱ��________(������������û����)����������
��3����֪����2CH3OH(g)![]() CH3OCH3(g)��H2O(g)
CH3OCH3(g)��H2O(g)
��CO(g)��2H2(g)![]() CH3OH(g)
CH3OH(g)
��CO(g)��H2O(g) ![]() CO2(g)��H2(g)
CO2(g)��H2(g)
ij�¶���������Ӧ��ƽ�ⳣ����ֵ����ΪK1��K2��K3������¶��·�Ӧ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g)�Ļ�ѧƽ�ⳣ��K��________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)����ij�̶�������ܱ������м���3 mol CO��3 mol H2����ַ�Ӧ��ָ���ԭ�¶ȣ��ⶨ������ѹǿΪ��Ӧǰ��1/2����CO��ת����Ϊ_________��
CH3OCH3(g)��CO2(g)�Ļ�ѧƽ�ⳣ��K��________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)����ij�̶�������ܱ������м���3 mol CO��3 mol H2����ַ�Ӧ��ָ���ԭ�¶ȣ��ⶨ������ѹǿΪ��Ӧǰ��1/2����CO��ת����Ϊ_________��
���𰸡���1���� ��С ��ac ��H2SeO3+2SO2+H2O=Se��+2H2SO4
��2����ad ��û�� ��3��K1K22K3 75%
��������
�����������1����������ʵĵ���Ϊ���ȹ��̣��������Ũ��һ��ʱ�������¶ȣ����Ƶ��룬��Kֵ��С��
��a�������������ǿ��̼�����ƣ�������̼�ᣬ��������CO2ͨ�����������Һ�з�Ӧ�����ӷ���ʽΪClO��+ H2O + CO2 = HClO + HCO3��������b�������������ǿ��̼�ᣬ��������SO2ͨ��̼������Һ�з�Ӧ�����ӷ���ʽΪSO2��H2O��2CO32��= 2HCO3����SO32������ȷ��c����ͬ�¶�ʱ�������ʵ���Ũ����������������NaOH��Һ��ȫ�к�����NaOH�����Ϊ��V(H2CO3����V(H2SO3����V(HClO��������d���ݵ��볣����֪����ǿ��˳����NaHSO3��HClO��NaHCO3����Խ����Ӧ����Խ����ˮ�⣬����Խǿ������ͬ�¶�ʱ����pH��������Һ�����ʵ���Ũ�ȹ�ϵc(Na2CO3)��c(NaClO)��c(Na2SO3)����ȷ��ѡac����������(H2SeO3����һ�ֶ�Ԫ���ᣬ�н�ǿ�������ԡ�����������Һ�в���ͨ��SO2��������ɫ���ʣ���˵�����߷�����������ԭ��Ӧ��SO2������Ϊ���ᣬ�����ᱻ��ԭΪ���������÷�Ӧ�Ļ�ѧ����ʽΪH2SeO3 + 2SO2+H2O��Se��+ 2H2SO4��
��2����a��ƽ�ⳣ��ֻ���¶��й�ϵ�����ƽ��������Ӧ������У���ƽ�ⳣ��Kֵ���Բ��ı䣬�������ͨ������������Ũ�ȣ���ȷ��b���ﵽ��ƽ��CrO42��������������Cr2O72�����������ʵ�2��������c���ﵽ��ƽ�����ҺpH��������Ҳ���ܼ�С������d��ƽ�����淴Ӧ�����ƶ����ٴ�ƽ��ǰ�淴Ӧ����һ����������Ӧ���ʣ���ȷ��ѡad���ڵ�c(Cr3+������10��3 mol��L��1����Һ��pH����4ʱ����Һ����������Ũ����10��10mol/L����ʱŨ���̣�10��3��(10��10��3��10��33��10��32�����û�г���������
��3����֪����2CH3OH(g)![]() CH3OCH3(g)��H2O(g)����CO(g)��2H2(g)
CH3OCH3(g)��H2O(g)����CO(g)��2H2(g)![]() CH3OH(g)����CO(g)��H2O(g)
CH3OH(g)����CO(g)��H2O(g) ![]() CO2(g)��H2(g)��ij�¶���������Ӧ��ƽ�ⳣ����ֵ����ΪK1��K2��K3������+�ڡ�2+�����õ����¶��µķ�Ӧ3CO(g)��3H2(g)
CO2(g)��H2(g)��ij�¶���������Ӧ��ƽ�ⳣ����ֵ����ΪK1��K2��K3������+�ڡ�2+�����õ����¶��µķ�Ӧ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g)����ѧƽ�ⳣ��K��K1K22K3��
CH3OCH3(g)��CO2(g)����ѧƽ�ⳣ��K��K1K22K3��
3CO(g�� +3H2(g�� ![]() CH3OCH3(g�� +CO2(g��
CH3OCH3(g�� +CO2(g��
��ʼ����mol�� 3 3 0 0
ת������mol�� 3x 3x x x
ƽ������mol��3��3x 3��3x x x
����6-4x��:6=1:2�����x��0.75����CO��ת����Ϊ75%��

����Ŀ����1��Ԫ�����ڱ���Ϊ �����У��� �����ڣ����ڱ����� �����У��� ���塣��ÿ��1�֣�
��2��д���������ʵĵ���ʽ����CO2 ����NaOH ����ÿ��2�֣�
��3���±���Ԫ�����ڱ���һ���֣���Ա��е���������Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺�����ӷ���ʽ2�֣�����ÿ��1�֣�
���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
�� | �� | �� | ||||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� | �� |
�� ����ЩԪ��������õķǽ���Ԫ���� ��
�� Ԫ�ص�����������Ӧ��ˮ������������ǿ���� ��������ǿ���� ��
�� �����Ե����������� �������������������ˮ���ﷴӦ�����ӷ���ʽΪ ��
�� ��������Ԫ���У�ԭ�Ӱ뾶������ ��
����Ŀ����1������ȷ��������:
��12C��13C��14C�� ������������; ��H2O��D2O����CH3CH2OH��CH3OCH3�� ��CH4��CH3CH3 ��
��Ϊͬλ�ص��� �� ��Ϊͬ����������� ��
��Ϊͬ���칹����� �� ��Ϊͬϵ�����
��2����A��B��C��D���ֽ������±���װ�ý���ʵ�顣
װ�� |
|
|
|
���� | ����A�� ���ܽ� | C���� ������ | A������ ����� |
����ʵ������ش��������⣺
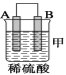
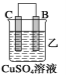
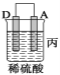
��װ�ü�������������_____ ___ ���A����B������
��װ������Һ��Cu2����_____ __���ƶ����B����C������
��װ�ñ��н���A�ϵ缫��Ӧ����___ ___���������Ӧ����ԭ��Ӧ������
�����ֽ��������ǿ������˳����____ ___��