��Ŀ����
����Ŀ�����й������ʽṹ�������У������������
��CH3COOH������̼ԭ�ӵ��ӻ�������sp2��sp3����
��Ԫ��Geλ�����ڱ���������IVA�壬��������Ų�ʽΪ[Ar]4s24p2������P��
�۷Ǽ��Է����������и߶ȶԳ��ԣ���BF3��H2O2��CO2�����ķ���
�ܱ��д��ڼ��Թ��ۼ���������ֻ�ѧ��������
��Cu(OH)2��һ����ɫ��״���������������ᡢ��ˮ��Ҳ����������������Һ��
������̬��HgCl2�����磬HgCl2ϡ��Һ�����ĵ�������˵����̬HgCl2�ǹ��ۻ����Ϊ�ǵ����
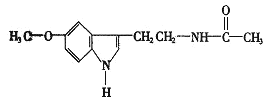
�߰�ˮ�д�NH3��H2O��������á�����������ʾ����ϳ�NH3��H2O���ӣ����ݰ�ˮ�����ʿ�֪NH3��H2O�Ľṹʽ�ɼ�Ϊ��
A.4��B.5��C.6��D.7��
���𰸡�A
��������
�ټ���̼ԭ�ӣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ���=4+0=4������Ϊsp3�ӻ���-COOH�У�̼ԭ�Ӽ۲���ӶԸ���=3+0=3���γ�3���Ҽ����¶Ե��ӣ��ӻ���ʽΪsp2�ӻ�������ȷ��
��Ge�Ǣ�A�������Ԫ�أ����������Ų�ʽΪ[Ar]3d104s24p2������P��Ԫ�أ��ʴ���
��H2O2Ϊ���Է��ӣ���BF3��CO2Ϊ�Ǽ��Է��ӣ��ṹ�Գƣ��ʴ���
��������ǻ�ѧ�����ʴ���
��������ͭ�ܺ��ᷢ���кͷ�Ӧ�������ᣬ������ͭҲ�ܺͰ�ˮ��Ӧ������������ӣ�������������ʽ�Σ���ˮ��Һ���ܵ���������ӣ�������ͭ����������������Һ�У�����ȷ��
��HgCl2��ϡ��Һ�����ĵ���������˵����������ʣ��ʴ���
�����Ӧ�γ���X��H-Y��ʽ���У�X��Y������N��O��FԪ��֮һ�������������ֿ��ܣ�(1)H3N��H-O-H��(2)H2N-H��OH2������һˮ�ϰ��ɵ����NH4+��OH-������(1)�ṹ�Ǻ����ģ�����ȷ��
������Тڢۢܢ�4��ʴ�ѡA��

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�