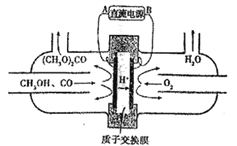
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΧΦΥαο»÷ς“Σ”Ο”Ύ≤ Βγœ‘œώΙήΒΡ”ΪΤΝ≤ΘΝßΚΆΧΊ÷÷≤ΘΝßΒ»ΒΡ÷Τ‘λΘ§Ά®≥Θ”…Χλ«ύ ·Ωσ(÷ς“Σ≥…Ζ÷ΈΣSrSO4Θ§ΜΙΚ§”–±ΒΒ»‘”÷ )÷Τ±ΗΘ§÷Τ±ΗΝς≥Χ»γΆΦΥυ ΨΘΚ
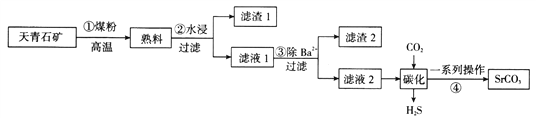
“―÷ΣΘΚiΘ°ο»‘ΎΓΑ¬Υ“Κ1Γ±ΓΑ¬Υ“Κ2Γ±÷–Ψυ÷ς“Σ“‘Sr(HS)2ΓΔSr(OH)2–Έ Ϋ¥φ‘Ύ
iiΘ°SrSO4ΓΔBaSO4ΒΡKSPΖ÷±π «3.2ΓΝ10-7ΓΔ1.0ΓΝ10-10
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)≤Ϋ÷ηΔΌΖ¥”ΠΘΚSrSO4+2C![]() SrS+2CO2ΓϋΘ§»τ‘Ύ±ξΉΦΉ¥Ωωœ¬≤ζ…ζ3.36 LCO2Θ§‘ρΉΣ“ΤΒγΉ” ΐΈΣ__________________ΓΘ
SrS+2CO2ΓϋΘ§»τ‘Ύ±ξΉΦΉ¥Ωωœ¬≤ζ…ζ3.36 LCO2Θ§‘ρΉΣ“ΤΒγΉ” ΐΈΣ__________________ΓΘ
(2)–¥≥ω≤Ϋ÷ηΔΎ÷–ΓΑΥ°ΫΰΓ±÷ς“ΣΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ________________________ΓΘ≤Ϋ÷ηΔΎΓΑΙΐ¬ΥΓ±≤ΌΉς–η“ΣΒΡ÷ς“Σ≤ΘΝß“«ΤςΈΣ________________________ΓΘ
(3)…œ ω…ζ≤ζΙΛ“’ΒΡ”≈Βψ «÷ ΝΩΚΟΓΔ≥…±ΨΒΆΘ§ΒΪ¥”ΜΖ±ΘΫ«Ε»ΩΦ¬«ΗΟΙΛ“’…ζ≤ζ¥φ‘ΎΟςœ‘ΒΡ»±Βψ «______________________________________________________ΓΘ
(4)»τœρΚ§”–Sr2+ΓΔBa2+ΒΡΓΑ¬Υ“Κ1Γ±÷–ΒΈΦ”œΓΝρΥαΘ§Β±ΝΫ÷÷≥ΝΒμΙ≤¥φ ±Θ§c(Sr2+)ΘΚc(Ba2+)=________________ΓΘ
(5)–¥≥ωΓΑΧΦΜ·Γ±Ιΐ≥Χ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_______________________________ΓΘ
(6)Ρ≥…ζ≤ζΤσ“Β”Οa kgΒΡΧλ«ύ ·Ωσ(Κ§SrSO4ΘΚ40ΘΞ)÷Τ±ΗΘ§Ήν÷’ΒΟΒΫb kgΧΦΥαο»≤ζΤΖΘ§≤ζ¬ ΈΣ______________(”ΟΚ§aΓΔbΒΡ ΫΉ”±μ Ψ)ΓΘ
ΓΨ¥πΑΗΓΩ 0.6NA ![]() …’±≠ΓΔ¬©ΕΖΓΔ≤ΘΝßΑτ ≤ζ…ζΈέ»ΨΈοH2SΤχΧεΓΔ≤ζ…ζΈέ»ΨΈοSO2ΤχΧεΘ®ΚœάμΦ¥Ω…Θ© 320
…’±≠ΓΔ¬©ΕΖΓΔ≤ΘΝßΑτ ≤ζ…ζΈέ»ΨΈοH2SΤχΧεΓΔ≤ζ…ζΈέ»ΨΈοSO2ΤχΧεΘ®ΚœάμΦ¥Ω…Θ© 320 ![]() Μρ
Μρ![]() ΓΔ
ΓΔ![]()
![]() ΓΝ100%
ΓΝ100%
ΓΨΫβΈωΓΩ(1)≤Ϋ÷ηΔΌΖ¥”ΠΘΚSrSO4+2C![]() SrS+2CO2ΓϋΘ§»τ‘Ύ±ξΉΦΉ¥Ωωœ¬≤ζ…ζ3.36 LCO2Θ§Έο÷ ΒΡΝΩΈΣ0.15molΘ§Ζ¥”Π÷–CΒΡΜ·ΚœΦέΙ≤…ΐΗΏ8Θ§‘ρ≤ζ…ζ0.15mol CO2Θ§ΉΣ“ΤΒγΉ” ΐ0.6molΘ§Ι ¥πΑΗΈΣΘΚ0.6NAΘΜ
SrS+2CO2ΓϋΘ§»τ‘Ύ±ξΉΦΉ¥Ωωœ¬≤ζ…ζ3.36 LCO2Θ§Έο÷ ΒΡΝΩΈΣ0.15molΘ§Ζ¥”Π÷–CΒΡΜ·ΚœΦέΙ≤…ΐΗΏ8Θ§‘ρ≤ζ…ζ0.15mol CO2Θ§ΉΣ“ΤΒγΉ” ΐ0.6molΘ§Ι ¥πΑΗΈΣΘΚ0.6NAΘΜ
(2) Χλ«ύ ·ΩσΗΏΈ¬œ¬”κΧΦΖ¥”Π…ζ≥…SrSΘ§ο»‘ΎΓΑ¬Υ“Κ1Γ±÷–÷ς“Σ“‘Sr(HS)2ΓΔSr(OH)2–Έ Ϋ¥φ‘ΎΘ§“ρ¥Υ≤Ϋ÷ηΔΎ÷–ΓΑΥ°ΫΰΓ±ΒΡ÷ς“ΣΖ¥”ΠΜ·―ßΖΫ≥Χ ΫΈΣ2SrS+2H2O= Sr(HS)2+Sr(OH)2ΓΘ≤Ϋ÷ηΔΎΓΑΙΐ¬ΥΓ±≤ΌΉς–η“ΣΒΡ÷ς“Σ≤ΘΝß“«Τς”–…’±≠ΓΔ¬©ΕΖΓΔ≤ΘΝßΑτΘ§Ι ¥πΑΗΈΣΘΚ2SrS+2H2O= Sr(HS)2+Sr(OH)2ΘΜ…’±≠ΓΔ¬©ΕΖΓΔ≤ΘΝßΑτΘΜ
(3) Sr(HS)2»ή“Κ÷–Ά®»κΕΰ―θΜ·ΧΦΜαΖ≈≥ωΝρΜ·«βΒ»Έέ»ΨΩ’ΤχΒΡΈο÷ Θ§Ι ¥πΑΗΈΣΘΚ≤ζ…ζΈέ»ΨΈοH2SΤχΧεΘΜ
(4)»τœρΚ§”–Sr2+ΓΔBa2+ΒΡΓΑ¬Υ“Κ1Γ±÷–ΒΈΦ”œΓΝρΥαΘ§Β±ΝΫ÷÷≥ΝΒμΙ≤¥φ ±Θ§c(Sr2+)ΘΚc(Ba2+)=![]() =
=![]() =
=![]() =3200Θ§Ι ¥πΑΗΈΣΘΚ3200ΘΜ
=3200Θ§Ι ¥πΑΗΈΣΘΚ3200ΘΜ
(5)ΓΑΧΦΜ·Γ±Ιΐ≥Χ÷–Sr(HS)2ΚΆSr(OH)2”κΕΰ―θΜ·ΧΦΖ¥”Π…ζ≥…SrCO3ΚΆH2SΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣSr(HS)2+Sr(OH)2+2CO2=2SrCO3Γΐ+2H2SΓϋΘ§Ι ¥πΑΗΈΣΘΚSr(HS)2+Sr(OH)2+2CO2 =2SrCO3Γΐ+2H2SΓϋΘΜ
(6)ΗυΨίSr‘ΣΥΊ ΊΚψΘ§SrSO4~ SrCO3Θ§ΧΦΥαο»ΒΡΈο÷ ΒΡΝΩ=![]() Θ§“ρ¥ΥΧΦΥαο»ΒΡ≤ζ¬ =
Θ§“ρ¥ΥΧΦΥαο»ΒΡ≤ζ¬ =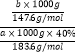 ΓΝ100%=
ΓΝ100%=![]() ΓΝ100%Θ§Ι ¥πΑΗΈΣΘΚ
ΓΝ100%Θ§Ι ¥πΑΗΈΣΘΚ![]() ΓΝ100%ΓΘ
ΓΝ100%ΓΘ

ΓΨΧβΡΩΓΩΗυΨί“Σ«σΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΓΘ
(1)ΗΏ¬·ΝΕΧζ «“±ΝΕΧζΒΡ÷ς“ΣΖΫΖ®Θ§ΖΔ…ζΒΡ÷ς“ΣΖ¥”ΠΈΣΘΚFe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g) H=-28.5kJ/molΘ§“±ΝΕΧζΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ±μ¥ο ΫK=____________Θ§Έ¬Ε»…ΐΗΏΚσΘ§K ÷Β________Θ®ΧνΓΑ‘ω¥σΓ±ΓΔΓΑ≤Μ±δΓ±ΜρΓΑΦθ–ΓΓ±Θ©ΓΘ
2Fe(s)+3CO2(g) H=-28.5kJ/molΘ§“±ΝΕΧζΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ±μ¥ο ΫK=____________Θ§Έ¬Ε»…ΐΗΏΚσΘ§K ÷Β________Θ®ΧνΓΑ‘ω¥σΓ±ΓΔΓΑ≤Μ±δΓ±ΜρΓΑΦθ–ΓΓ±Θ©ΓΘ
(2)“―÷ΣΘΚΔΌFe2O3(s)+3C( ·ΡΪ) = 2Fe(s)+3CO(g) H1= +489.0kJ/mol
ΔΎFe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g) H2= -28.5kJ/mol
2Fe(s)+3CO2(g) H2= -28.5kJ/mol
ΔέCΘ® ·ΡΪΘ©+ CO2(g) = 2CO(gΘ© H3= akJ/mol ,
‘ρa=__________kJ/molΓΘ
(3)‘ΎTΓφ ±Θ§Ζ¥”ΠFe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g)ΒΡΤΫΚβ≥Θ ΐK=64Θ§‘Ύ2LΚψ»ίΟή±’»ίΤς÷–Θ§Α¥œ¬±μΥυ ΨΦ”»κΈο÷ Θ§Ψ≠Ιΐ“ΜΕΈ ±ΦδΚσ¥οΒΫΤΫΚβΓΘ
2Fe(s)+3CO2(g)ΒΡΤΫΚβ≥Θ ΐK=64Θ§‘Ύ2LΚψ»ίΟή±’»ίΤς÷–Θ§Α¥œ¬±μΥυ ΨΦ”»κΈο÷ Θ§Ψ≠Ιΐ“ΜΕΈ ±ΦδΚσ¥οΒΫΤΫΚβΓΘ
Fe2O3 | CO | Fe | CO2 | |
ΦΧ§mol | 1.0 | 1.0 | 1.0 | 1.0 |
ΔΌΤΫΚβ ±CO ΒΡΉΣΜ·¬ ΈΣ_______ΓΘ
ΔΎœ¬Ν–«ιΩω±ξ÷ΨΖ¥”Π¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «________Θ®ΧνΉ÷ΡΗΘ©ΓΘ
aΘ°»ίΤςΡΎΤχΧεΟήΕ»±Θ≥÷≤Μ±δ
bΘ°»ίΤςΡΎΤχΧε―Ι«Ω±Θ≥÷≤Μ±δ
cΘ°COΒΡœϊΚΡΥΌ¬ ΚΆCO2ΒΡ…ζ≥…ΥΌ¬ œύΒ»
ΓΨΧβΡΩΓΩCrSiΓΔGe-GaAsΓΔZnGeAs2ΓΔΨέΏΝΩ©ΓΔΧΦΜ·ΙηΚΆ―θΜ·―«Ά≠ΕΦ «÷Ί“ΣΒΡΑκΒΦΧεΜ·ΚœΈοΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ΜυΧ§Ηθ‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ___________Θ§Τδ÷–Έ¥≥…Ε‘ΒγΉ” ΐΈΣ____________ΓΘ
(2) Ge-GaAs÷–‘ΣΥΊGeΓΔGaΓΔAsΒΡΒΎ“ΜΒγάκΡή¥”¥σΒΫ–ΓΒΡΥ≥–ρΈΣ_______________ΓΘZnGeAs2÷–ZnΓΔGeΓΔAsΒΡΒγΗΚ–‘¥”¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________________ΓΘ
(3)ΨέΏΝΩ©ΒΡΒΞΧεΈΣΏΝΩ©(![]() )Θ§ΗΟΖ÷Ή”÷–ΒΣ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–ΆΈΣ__________ΘΜΖ÷Ή”÷–Π“Φϋ”κΠ–ΦϋΒΡ ΐΡΩ÷°±»ΈΣ________________ΓΘ
)Θ§ΗΟΖ÷Ή”÷–ΒΣ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–ΆΈΣ__________ΘΜΖ÷Ή”÷–Π“Φϋ”κΠ–ΦϋΒΡ ΐΡΩ÷°±»ΈΣ________________ΓΘ
(4)ΧΦΜ·ΙηΓΔΨßΧεΙηΦΑΫπΗ’ ·ΒΡ»έΒψ»γœ¬±μΘΚ
ΝΔΖΫΧΦΜ·Ιη | ΨßΧεΙη | ΫπΗ’ · | |
»έΒψ/Γφ | 2973 | 1410 | 3550ΓΪ4000 |
Ζ÷Έω»έΒψ±δΜ·Ιφ¬…ΦΑΤδ≤ν“λΒΡ‘≠“ρΘΚ__________________________________________________ΓΘ
(5)―θΜ·―«Ά≠ΒΡ»έΒψΈΣ1235ΓφΘ§ΤδΙΧΧ§ ±ΒΡΒΞΨßΑϊ»γœ¬ΆΦΥυ ΨΓΘ
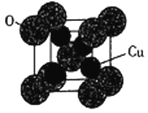
ΔΌ―θΜ·―«Ά≠ τ”Ύ__________ΨßΧεΓΘ
ΔΎ“―÷ΣCu2OΒΡΨßΑϊ≤Έ ΐa=425.8pm,‘ρΤδΟήΕ»ΈΣ__________ gΓΛcm-3(Ν–≥ωΦΤΥψ ΫΦ¥Ω…)ΓΘ