��Ŀ����
����Ŀ��������������������������ص����������������ǵij��м���������˼����Σ�����о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
(1)�����Ƽ�ѭ�������ѳ������е�SO2��
�����Ƽ�ѭ�����У�Na2SO3��Һ��Ϊ����Һ������NaOH��Һ����SO2�Ƶã��÷�Ӧ�����ӷ���ʽ��___��
������Һ����SO2�Ĺ����У�pH��n(SO32-)��n(HSO3-)�仯��ϵ���±���
n(SO32-):n(HSO3-) | 91��9 | 1��1 | 9��91 |
pH | 8.2 | 7.2 | 6.2 |
���ϱ��жϣ�NaHSO3��Һ��______�ԣ���ᡱ��������С������û�ѧƽ��ԭ�����ͣ�__________��
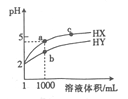
�۵�����Һ��pH����ԼΪ6ʱ����������������������ʾ��ͼ���£�
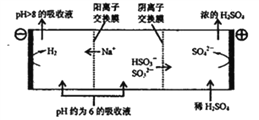
д��HSO3-�������ŵ�ĵ缫��Ӧʽ��____________��������������ҺpH����8����ʱ������Һ������ѭ�����á�
(2)��CH4����ԭNO2�������������������Ⱦ�����磺
CH4(g) + 4NO2(g) =4NO(g) + CO2(g) + 2H2O(g)�� ��H=-574 kJ mol-1
CH4(g) + 4NO(g) =2N2(g) + CO2(g) + 2H2O(g)����H=-1160 kJ mol-1
���ñ�״����4.48 L CH4��ԭ NO2��N2������������ת�Ƶĵ�������Ϊ______(�����ӵ�������ֵ��NA��ʾ)���ų�������Ϊ_________kJ��
(3)��ҵ�Ϻϳɰ������������Ʊ������У����е�һ����ӦΪ��
CO(g) + H2O(g) ![]() CO2(g) + H2(g)�� ��H<0
CO2(g) + H2(g)�� ��H<0
���������£���CO(g)��H2O(g)�����ױ�Ϊ1:2�����ܱ������з���������Ӧ���ﵽƽ��ʱ���CO(g)��H2O(g)�����Ϊح��6����ƽ�ⳣ��K=__________(������������λС��)��
���𰸡� 2OH-+SO2�TH2O + SO32- �� HSO3-���ڣ�HSO3-![]() H++ SO32-�� HSO3-+H2O
H++ SO32-�� HSO3-+H2O![]() H2SO3+OH-, HSO3-�ĵ���̶�ǿ��ˮ��̶� HSO3-+H2O-2e-�TSO42-+3H+ l.6NA 173.4 2.67
H2SO3+OH-, HSO3-�ĵ���̶�ǿ��ˮ��̶� HSO3-+H2O-2e-�TSO42-+3H+ l.6NA 173.4 2.67
����������1��������������ͼӦ�����κ�ˮ�����Զ���������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ����ʽΪ2OH-+SO2��H2O + SO32-��������Һ����Ҫ��HSO3-���ڣ�HSO3-�ĵ������������n��SO32-����n��HSO3-����1��1�����ݱ���֪����������������ӵ����ʵ�����������������ӵ����ʵ���ʱ��������������Һ�����ԣ�������������Ӽ���ˮ�����ܵ��룬������������Һ������ͬʱ˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ����������������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ��缫��ӦʽΪ��HSO3-+H2O-2e-��SO42-+3H+����2����֪��
��CH4(g) + 4NO2(g) =4NO(g) + CO2(g) + 2H2O(g)�� ��H=-574 kJ mol-1
��CH4(g) + 4NO(g) =2N2(g) + CO2(g) + 2H2O(g)�� ��H=-1160 kJ mol-1
���ݸ�˹���ɽ���+��/2���ɵ�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867kJ/mol��n��CH4��=4.48L��22.4L/mol������������ת�Ƶĵ�������Ϊ��0.20mol��8NA=1.60NA���ų�������Ϊ��0.2mol��867kJ/mol=173.4kJ����3��ͬһ�����и���������ʵ���֮�ȵ��������֮�ȣ������������Ϊ1L�������һ����̼�����ʵ���Ϊ1mol����ˮ�����ʵ���Ϊ2mol������ƽ��ʱһ����̼�����ʵ���Ϊx����
CO��g��+H2O��g��CO2��g��+H2��g��
��ʼ����mol��1 2 0 0
ת������mol��1-x 2-6x 1-x 1-x
ƽ������mol��x 6x 1-x 1-x
��1-x������2-6x��=1��1��x=0.2mol������ƽ��ʱ��c��CO��=0.2mol/L��c��H2O��=1.2mol/L��c��H2��=c��CO2��=0.8mol/L��K=0.8��0.8/0.2��1.2=8/3��2.67��

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�