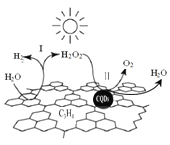
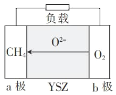
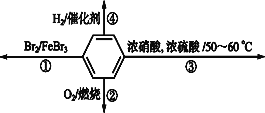
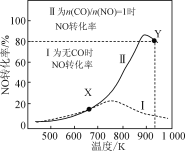
��Ŀ����
����Ŀ���а��ֶ���������Ԫ��x��y��z��d��e��f��g��h������x��y��d��f����ԭ�������ĵ�������ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯��ͼ1��ʾ�� z��e��g��h������������Ӧˮ������Һ(Ũ�Ⱦ�Ϊ0.01mol/L)��pH��ԭ�������Ĺ�ϵ��ͼ2��ʾ��
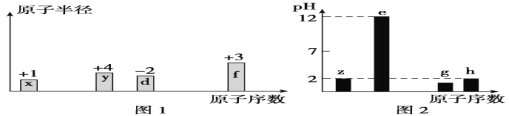
����������Ϣ�����жϣ�����˵����ȷ�ǣ� ��
A.d��e��f��g �γɵļ������У��뾶������d����
B.d��e�γɵĻ�������ֻ�������Ӽ�
C.x��y��z��d��e��f��g��h�ĵ����У�f���۵����
D.x��y�����γɶ��ֻ�������ܴ��ڷǼ��Թ��ۼ�
���𰸡�D
��������
x��y��z��d��e��f��g��hΪԭ���������ε����Ķ���������Ԫ�أ���ͼ�еĻ��ϼۡ�ԭ�Ӱ뾶�Ĵ�С��ԭ������������֪��x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�f��AlԪ�أ�z��e��g��h������������Ӧˮ������ҺŨ�Ⱦ�Ϊ0.01mol��L-1 ��e��pHΪ12��ΪһԪǿ���e��NaԪ�أ�z��h��pH��Ϊ2��ΪһԪǿ�ᣬ��zΪNԪ�ء�hΪClԪ�أ�g��pHС��2����g��Ϊ��Ԫǿ�ᣬ��gΪSԪ�أ��ݴ˷���������⡣
A��d��e��f��g �γɵļ����ӷֱ�Ϊ��O2-��Na+��Al3+��S2-��O2-��Na+��Al3+���ӵĵ��Ӳ�����ͬ�����˵����Al>Na>O�������Ӱ뾶O2->Na+>Al3+����S2-�ĵ��Ӳ�����࣬�뾶��ʰ뾶������g���ӣ�Aѡ�����
B��d��e�γɵĻ����������Na2O��Ҳ������Na2O2��Na2O2�Ⱥ������Ӽ���Ҳ���й��ۼ���Bѡ�����
C��x��y��z��d��e��f��g��h�ĵ��ʷֱ�Ϊ��H2��C��N2��O2��Na��Al��S��Cl2��C���ʿ��γɽ��ʯ��Ϊԭ�Ӿ��壬�ʵ����۵���ߵĿ�����y��Cѡ�����
D��x��y�����γɶ����л����������CH2=CH2�ȴ����ŷǼ��Լ���Dѡ����ȷ��
��ѡD��

����Ŀ��������������C9H10O2������Ϊ��Ϣ��������������һ����ɫ��Һ�壬������ˮ������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��壬�ܼ��ȡ����Ʊ�����Ϊ��
��֪��
���� | ��Է������� | ��ɫ��״̬ | �е�(��) | �ܶ�(g��cm-3) |
������ | 122 | ��ɫƬ״���� | 249 | 1.2659 |
���������� | 150 | ��ɫ����Һ�� | 212.6 | 1.05 |
�Ҵ� | 46 | ��ɫ����Һ�� | 78.3 | 0.7893 |
������ | 84 | ��ɫ����Һ�� | 80.8 | 0.7318 |
��������100���Ѹ��������
ʵ�鲽�����£�
����Բ����ƿ�м���12.20 g�����ᣬ25 mL 95%���Ҵ�����������20 mL�������Լ�4 mLŨ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ�������������¶���65��70����Ȼ���2 h�����÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
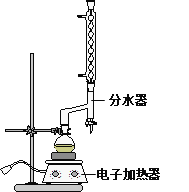
�ڷ�Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
�۽���ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�������ԡ��÷�Һ©���ֳ��л��㣬ˮ����25 mL������ȡ��Һ��Ȼ��ϲ����л��㣬�����Ȼ��ƣ����ã����ˣ�����Һ�����������������Ѻͻ�����������£�����210��213�����֡�
�ܼ���ϸ�ò�Ʒ���Ϊ12.86 mL��
�ش��������⣺
��1���ڸ�ʵ���У�Բ����ƿ���ݻ����ʺϵ���_________��������ȷѡ��ǰ����ĸ����
A��25 mL B��50 mL C��100 mL D��250 mL
��2���������ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����_____________________��
��3���������Ӧ������ֵ��¶���____________��
A��65��70�� B��78��80�� C��85��90�� D��215��220��
��4������ۼ���Na2CO3��������______________________����Na2CO3���벻�㣬��֮������ʱ��������ƿ�пɼ����������ɣ������������ԭ����____________��
��5�����ڲ�����е���ȡ��Һ����������ȷ����__________��
A��ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ���������Һ©����ת������������ҡ
B����ҡ���κ����Һ©���ϿڵIJ���������
C����������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D���ų�Һ��ʱ��Ӧ���Ͽڲ������������ϵİ��۶�©�����ϵ�С��
��6�����㱾ʵ��IJ���Ϊ_______________��