��Ŀ����
��ҵ���Ի�ͭ��(��Ҫ�ɷ�CuFeS2)Ϊԭ���Ʊ�����ͭ�����������ֹ��ա�
I������������:���������Ļ�ͭ�����ʯӢ����ͨ�˿������б��գ������Ƶô�ͭ��
��1�����յ��ܷ�Ӧʽ�ɱ�ʾΪ��2CuFeS2 + 2SiO2+5O2��2Cu+2FeSiO3+4SO2�÷�Ӧ���������� ��
��2�����д���SO2�ķ���������������_____
A�߿��ŷ� B�ô�����Һ�����Ʊ���������
C�ð�ˮ���պ��پ������Ʊ������ D��BaCl2��Һ�����Ʊ�BaSO3
��3��¯����Ҫ�ɷ���FeO ��Fe2O3 ��SiO2��Al2O3�ȣ�Ϊ�õ�Fe2O3�������ܽ��������������δ�漰���IJ����� ��
A���� B�ӹ���NaOH��Һ C�����ᾧ D���� E��������
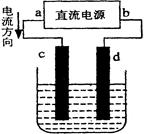
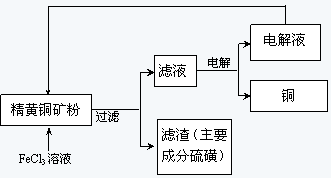
II��FeCl3��Һ��ȡ����:��������������ͼ��ʾ
��4�����������У�CuFeS2��FeCl3��Һ��Ӧ�����ӷ���ʽΪ ____________��
��5���ù��������У�����ѭ�����õ�������____ ���ѧʽ����
��6������ʯī�缫�����Һ��д�������ĵ缫��ʽ_____________��
��7����ͭ���к�����Pb,����C1һŨ�ȿɿ�����Һ��Pb2+��Ũ�ȣ���c(C1һ)��2mo1��L-1ʱ��Һ��Pb2+���ʵ���Ũ��Ϊ mol��L-1��[��֪KSP(PbCl2)��1 x 10һ5]
I������������:���������Ļ�ͭ�����ʯӢ����ͨ�˿������б��գ������Ƶô�ͭ��
��1�����յ��ܷ�Ӧʽ�ɱ�ʾΪ��2CuFeS2 + 2SiO2+5O2��2Cu+2FeSiO3+4SO2�÷�Ӧ���������� ��
��2�����д���SO2�ķ���������������_____
A�߿��ŷ� B�ô�����Һ�����Ʊ���������
C�ð�ˮ���պ��پ������Ʊ������ D��BaCl2��Һ�����Ʊ�BaSO3
��3��¯����Ҫ�ɷ���FeO ��Fe2O3 ��SiO2��Al2O3�ȣ�Ϊ�õ�Fe2O3�������ܽ��������������δ�漰���IJ����� ��
A���� B�ӹ���NaOH��Һ C�����ᾧ D���� E��������
II��FeCl3��Һ��ȡ����:��������������ͼ��ʾ

��4�����������У�CuFeS2��FeCl3��Һ��Ӧ�����ӷ���ʽΪ ____________��
��5���ù��������У�����ѭ�����õ�������____ ���ѧʽ����
��6������ʯī�缫�����Һ��д�������ĵ缫��ʽ_____________��
��7����ͭ���к�����Pb,����C1һŨ�ȿɿ�����Һ��Pb2+��Ũ�ȣ���c(C1һ)��2mo1��L-1ʱ��Һ��Pb2+���ʵ���Ũ��Ϊ mol��L-1��[��֪KSP(PbCl2)��1 x 10һ5]
��1��CuFeS2��O2
��2��A��D
��3��C
��4��CuFeS2+4Fe3+��Cu2++5Fe2++2S
��5��FeCl3��
��6��Fe2+-e����Fe3+��
��7��2.5��10��6
��2��A��D
��3��C
��4��CuFeS2+4Fe3+��Cu2++5Fe2++2S
��5��FeCl3��
��6��Fe2+-e����Fe3+��
��7��2.5��10��6
��1�����ݷ���ʽ2CuFeS2 + 2SiO2+5O2��2Cu+2FeSiO3+4SO2֪��ͭԪ�صĻ��ϼ۽��ͣ���Ԫ�صĻ��ϼ۽��ͣ���˸÷�Ӧ����������CuFeS2��O2��
��2��SO2�Ǵ�����Ⱦ�A�����ô�����Һ�����Ʊ��������ƣ����Է�ֹ��Ⱦ��B��ȷ���ð�ˮ���պ�������������泥�Ҳ���Է�����Ⱦ��C��ȷ��SO2���������Ȼ�����Һ��D������ѡAD��
��3��¯����Ҫ�ɷ���FeO��Fe2O3��SiO2��Al2O3�ȣ�Ϊ�õ�Fe2O3���������ܽ����Ҫ���˳��������裬Ȼ���ټ�������������Һ�е�������������Ϊ���������ӣ��ټ����������������Һ����Ҫ�ɷ��������������������ˡ�ϴ�ӡ����վͿ��Եõ�����������������δ�漰��ʵ������������ᾧ��
��4�����˵õ�����ֽ�к������ʣ���˵��CuFeS2��FeCl3��Һ����������ԭ��Ӧ����CuFeS2+4Fe3+��Cu2++5Fe2++S��
��5�����ʱ�����ĵ��Һ�ֿ������ͭ��Ӧ�����Ըù��������У�����ѭ�����õ�������FeCl3��
��6������������ʧȥ���ӣ��������ʯī���缫�����Һ������������������ʧȥ���ӣ�����������Ӧ��Fe2+��e����Fe3+��
��7����֪KSP(PbCl2)��1��10һ5����˵���Һ��c(C1һ)��2mo1��L-1ʱ��Һ��Pb2+���ʵ���Ũ��Ϊ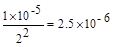 mol��L-1��
mol��L-1��
��2��SO2�Ǵ�����Ⱦ�A�����ô�����Һ�����Ʊ��������ƣ����Է�ֹ��Ⱦ��B��ȷ���ð�ˮ���պ�������������泥�Ҳ���Է�����Ⱦ��C��ȷ��SO2���������Ȼ�����Һ��D������ѡAD��
��3��¯����Ҫ�ɷ���FeO��Fe2O3��SiO2��Al2O3�ȣ�Ϊ�õ�Fe2O3���������ܽ����Ҫ���˳��������裬Ȼ���ټ�������������Һ�е�������������Ϊ���������ӣ��ټ����������������Һ����Ҫ�ɷ��������������������ˡ�ϴ�ӡ����վͿ��Եõ�����������������δ�漰��ʵ������������ᾧ��
��4�����˵õ�����ֽ�к������ʣ���˵��CuFeS2��FeCl3��Һ����������ԭ��Ӧ����CuFeS2+4Fe3+��Cu2++5Fe2++S��
��5�����ʱ�����ĵ��Һ�ֿ������ͭ��Ӧ�����Ըù��������У�����ѭ�����õ�������FeCl3��
��6������������ʧȥ���ӣ��������ʯī���缫�����Һ������������������ʧȥ���ӣ�����������Ӧ��Fe2+��e����Fe3+��
��7����֪KSP(PbCl2)��1��10һ5����˵���Һ��c(C1һ)��2mo1��L-1ʱ��Һ��Pb2+���ʵ���Ũ��Ϊ
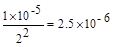 mol��L-1��
mol��L-1��
��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
�����Ŀ

 �����ӣ��缫Ϊ���Ե缫��������������ȷ����
�����ӣ��缫Ϊ���Ե缫��������������ȷ����