��Ŀ����
3�� ��1�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��
��1�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ����a����ͨ��״���£����ʯ��ʯī��Ƚ�ʯī��������ʯ����ʯī�������ȶ���ʯī��ȼ����Ϊ-393.5kJ?mol-1��
��b��12gʯī��һ����������ȼ�գ���������36g���ù��̷ų�������Ϊ252.0kJ��
��2����֪��N2��O2�����л�ѧ���ļ��ֱܷ���946kJ?mol-1��497kJ?mol-1��N2��g��+O2��g���T2NO��g����H=+180.0kJ?mol-1��NO�����л�ѧ���ļ���Ϊ631.5kJ?mol-1��
��3���ۺ������й���Ϣ����д����CO��ȥNO���Ȼ�ѧ����ʽ��2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.0kJ?mol-1��
���� ��1����a��������������Խ�ߣ�����Խ���ȶ�������ͼ���ж�ʯī��ȼ���ȣ�
��b�����ݼ�ֵ�����ж����ɵ�������ɣ�����Ȼ�ѧ����ʽ����õ���
��2���ݡ�H=��Ӧ����ܺ�-��������ܺ����㣻
��3�����ø�˹���ɽ����֪�Ȼ�ѧ����ʽ���㷴Ӧ�ȣ���д���Ȼ�ѧ����ʽ��
��� �⣺��1����a��ͼ��������ʯ��������ʯī������Խ��Խ�ȶ�������˵��ʯī�ȶ���ͼ�����1molʯī��ȫȼ������1mol������̼�ų�������Ϊ393.5kJ����ʯī��ȼ����Ϊ��H=-393.5kJ?mol-1��
�ʴ�Ϊ��ʯī��-393.5kJ?mol-1��
��b��12gʯī���ʵ���Ϊ1mol����һ����������ȼ�գ�����Ԫ���غ㣬�����ɶ�����̼����Ϊ44g��������һ����̼����Ϊ28g����������36g��28g��36g��44g���ж����ɵ�����Ϊһ����̼�Ͷ�����̼���壬��һ����̼���ʵ���Ϊx��������̼���ʵ���Ϊ��1-x��mol��28x+44��1-x��=36g��x=0.5mol��������̼���ʵ���Ϊ0.5mol������ͼ�������C��ʯī��s��+O2��g���TCO2��g����H=-393.5 kJ•mol-1 ��C��ʯī��s��+$\frac{1}{2}$O2��g���TCO��g����H=-110.5 kJ•mol-1
���ɶ�����̼��һ����̼�������ų�����=393.5 kJ•mol-1 ��0.5mol+110.5 kJ•mol-1 ��0.5mol=252KJ��12gʯī��һ����������ȼ�գ���������36g���ù��̷ų�������Ϊ252.0kJ��
�ʴ�Ϊ��252.0 kJ��
��2����H=��Ӧ����ܺ�-��������ܺͣ�946kJ/mol+497kJ/mol-2Q��N-O��=180.0kJ/mol��Q��N-O��=631.5KJ/mol���ʴ�Ϊ��631.5��
��3����֪��C��ʯī��s��+O2��g��=CO2��g����H=-393.5 kJ•mol-1��
��C��ʯī��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.5 kJ•mol-1��
��N2��g��+O2��g��=2NO��g����H=+180kJ•mol-1��
�ɸ�˹���ɣ�����ʽ�١�2-�ڡ�2-�۵� 2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.0kJ?mol-1��
�ʴ�Ϊ��2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.0kJ?mol-1��
���� ���⿼���˻�ѧ��Ӧ�������仯�뷴Ӧ���ʱ��ϵ�ķ����жϣ�ͼ����ۺ�Ӧ�ã�����ͼ���ʱ�ͻ�ܼ��㷽���������ǽ���Ĺؼ���

| A�� | ϡ���� | B�� | ����������Һ | C�� | ����ͭ��Һ | D�� | ʳ��ˮ |
d Fe3++e Br2+f Cl-������ѡ���е����������ӷ���ʽ�е�a��b��c��d��e��fһһ��Ӧ�����в����Ϸ�Ӧʵ�ʵ��ǣ�������
| A�� | 2 4 3 2 2 6 | B�� | 0 2 1 0 1 2 | ||
| C�� | 2 0 1 2 0 2 | D�� | 2 2 2 2 1 4 |
| A�� | 62 g | B�� | 78mol?L-1 | C�� | 78g?mol-1 | D�� | 62mol |
| A�� | ���� | |
| B�� | ����100 mL 0.1 mol•L-1�Ĵ�����Һ | |
| C�� | ����������0.5 mol•L-1������ | |
| D�� | ����������1 mol•L-1��NaOH��Һ |
| A�� | 5.0 L | B�� | 8.9 L | C�� | 15.7 L | D�� | 26.9 L |
| A�� | 0.1mol•L-1 NaOH��Һ��Na+����ĿΪ0.1NA | |
| B�� | ��״���£�2.24 LCCl4�еķ�����ĿΪ0.1NA | |
| C�� | 0.1mol Na2O2������CO2��Ӧת�Ƶĵ�����ĿΪ0.1NA | |
| D�� | 3.2gO2��O3�Ļ���ﺬ�еķ�����ĿΪ0.1NA |
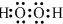 ��
��