��Ŀ����
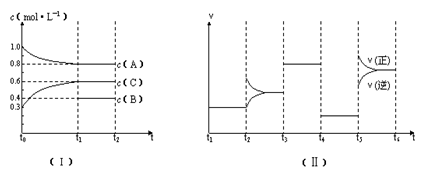
��10�֣�.��һ��������ܱ������м���2 mol A��0.6 mol C��һ������B�������塣һ�������·�����Ӧ�������ʵ���Ũ����ʱ��仯��ͼ������ʾ������t0--t1��c��B��δ������ͼ����Ϊt2ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯 ��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3---t4��Ϊʹ�ô�����
��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3---t4��Ϊʹ�ô�����
��ش��������⣺
��1����t1="15" min����t0---t1����C���ʵ�Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ mol�� L��1��min��1��
L��1��min��1��
��2��t4--t5�θı������Ϊ ��B����ʼ���ʵ���Ũ��Ϊ mol�� L��1��
��3��t5----t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01mol�����˹����е���ЧӦΪa kJ������д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ

 ��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3---t4��Ϊʹ�ô�����
��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ�ѹǿ���¶ȡ������е�һ������������t3---t4��Ϊʹ�ô����� ��ش��������⣺
��1����t1="15" min����t0---t1����C���ʵ�Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ mol��
 L��1��min��1��
L��1��min��1����2��t4--t5�θı������Ϊ ��B����ʼ���ʵ���Ũ��Ϊ mol�� L��1��
��3��t5----t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01mol�����˹����е���ЧӦΪa kJ������д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ


��1��0.02
��2����Сѹǿ 0.5
��3��2A(g)+B(g)="3C(g)"
��H="+200a " KJ/mol
��2����Сѹǿ 0.5
��3��2A(g)+B(g)="3C(g)"
��H="+200a " KJ/mol
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2C(g)�ﵽƽ��ı�־�ǣ� ��
2C(g)�ﵽƽ��ı�־�ǣ� �� 2Z(g) ��H��0��T��ʱ��ѧƽ�ⳣ��K = 2�����ܱ������з�Ӧ�ﵽƽ�⣬����˵����ȷ���ǣ� ��
2Z(g) ��H��0��T��ʱ��ѧƽ�ⳣ��K = 2�����ܱ������з�Ӧ�ﵽƽ�⣬����˵����ȷ���ǣ� �� mZ(g)����H����a kJmol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����
mZ(g)����H����a kJmol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����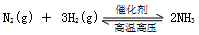 �Ļ�ѧƽ�ⳣ������ʽΪ
�Ļ�ѧƽ�ⳣ������ʽΪ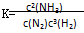
 2Z(g)����H<0���ڲ�ͬ�¶ȣ�T1��T2���£�����Z�����ʵ���n�뷴Ӧʱ��t�Ĺ�ϵ��ͼ��ʾ���������ж�����ȷ���ǣ� ��
2Z(g)����H<0���ڲ�ͬ�¶ȣ�T1��T2���£�����Z�����ʵ���n�뷴Ӧʱ��t�Ĺ�ϵ��ͼ��ʾ���������ж�����ȷ���ǣ� ��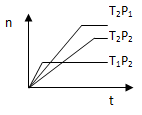
 pC(g)�ڲ�ͬ�¶�(T)��ѹǿ(P)�£�������C����������C%�ı仯��������й��ڸ�����Ӧ��ЧӦ������ʽ��A��B��C�Ļ�ѧ���������ж��У���ȷ���ǣ� ��
pC(g)�ڲ�ͬ�¶�(T)��ѹǿ(P)�£�������C����������C%�ı仯��������й��ڸ�����Ӧ��ЧӦ������ʽ��A��B��C�Ļ�ѧ���������ж��У���ȷ���ǣ� ��
 pC(g) ��H=?��Ӧ�����¼���±���
pC(g) ��H=?��Ӧ�����¼���±��� CO2��g��+H2��g����H��0���Իش���������
CO2��g��+H2��g����H��0���Իش��������� ��
�� ����ʼʱc��CO��="2" mol��L-1��c��H2O��="3" mol��L-1���ﵽƽ��ʱCO��ת����Ϊ60%�����ڴ��¶��£��÷�Ӧ��ƽ�ⳣ��K=
����ʼʱc��CO��="2" mol��L-1��c��H2O��="3" mol��L-1���ﵽƽ��ʱCO��ת����Ϊ60%�����ڴ��¶��£��÷�Ӧ��ƽ�ⳣ��K=