��Ŀ����
�����ĵ⻯��������·������вⶨ���䲽�����£�
�������Ի������Խ��������ù�����Br2���������е�I-������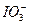 ��
��
����Т�������Һ�Գ�ȥ������Br2��Ȼ�������������¼��������KI��Һ����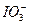 ��ԭΪI2��
��ԭΪI2��
���ڢ��мӵ�����ָʾ������Na2S2O3����Һ�ζ�(2Na2S2O3+I2====2NaI+Na2S4O6)��
ͨ����������ɲ�õ⻯����I-�ĺ�����
(1)д������٢��з�����Ӧ�����ӷ���ʽ��
��__________________________________________��
��__________________________________________��
(2)����Ʒ����1 mol I-����������Na2S2O3�����ʵ����Ƕ��٣�
(3)���ڲⶨʱ��ȷ��ȡ��KI����Ʒ��Һ25.00 mL���յ�ʱ����0.050 mol��L-1 Na2S2O3��Һ20.06 mL���Լ�����Ʒ��Һ��KI�ĺ���(g��L-1)��
�������Ի������Խ��������ù�����Br2���������е�I-������
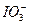 ��
������Т�������Һ�Գ�ȥ������Br2��Ȼ�������������¼��������KI��Һ����
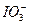 ��ԭΪI2��
��ԭΪI2�����ڢ��мӵ�����ָʾ������Na2S2O3����Һ�ζ�(2Na2S2O3+I2====2NaI+Na2S4O6)��
ͨ����������ɲ�õ⻯����I-�ĺ�����
(1)д������٢��з�����Ӧ�����ӷ���ʽ��
��__________________________________________��
��__________________________________________��
(2)����Ʒ����1 mol I-����������Na2S2O3�����ʵ����Ƕ��٣�
(3)���ڲⶨʱ��ȷ��ȡ��KI����Ʒ��Һ25.00 mL���յ�ʱ����0.050 mol��L-1 Na2S2O3��Һ20.06 mL���Լ�����Ʒ��Һ��KI�ĺ���(g��L-1)��
(1)��3Br2+I-+3H2O====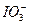 +6Br-+6H+
+6Br-+6H+
��6H++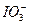 +5I-====3I2+3H2O
+5I-====3I2+3H2O
(2)6 mol (3)1.11 g��L-1
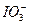 +6Br-+6H+
+6Br-+6H+��6H++
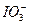 +5I-====3I2+3H2O
+5I-====3I2+3H2O(2)6 mol (3)1.11 g��L-1
���������д������٢��з��������ӷ�Ӧ����ʽ��
��3Br2+I-+3H2O====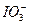 +6Br-+6H+
+6Br-+6H+
��6H++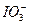 +5I-====3I2+3H2O
+5I-====3I2+3H2O
����2Na2S2O3+I2====2NaI+Na2S4O6�͢٢���ʽ��
I-��3I2��6Na2S2O3
1 mol 6 mol
n 0.050 mol��L-1��20.06��10-3 L
��ã�n=1.671��10-4 mol
������Ʒ��Һ��KI�ĺ���Ϊ1.671��10-4 mol��166 g��mol-1/25.00��10-3 L="1.11" g��L-1��
��3Br2+I-+3H2O====
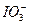 +6Br-+6H+
+6Br-+6H+��6H++
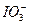 +5I-====3I2+3H2O
+5I-====3I2+3H2O����2Na2S2O3+I2====2NaI+Na2S4O6�͢٢���ʽ��
I-��3I2��6Na2S2O3
1 mol 6 mol
n 0.050 mol��L-1��20.06��10-3 L
��ã�n=1.671��10-4 mol
������Ʒ��Һ��KI�ĺ���Ϊ1.671��10-4 mol��166 g��mol-1/25.00��10-3 L="1.11" g��L-1��

��ϰ��ϵ�д�
�����Ŀ