��Ŀ����
��8�֣�ij��ѧ��ѧ�о���ѧϰС����������װ����ȡ��̽�����������ʡ�A�з�����Ӧ�Ļ�ѧ����ʽ��2NH4Cl + Ca(OH)2 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O

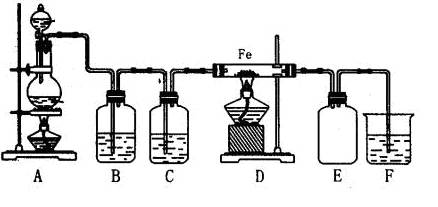
��1��A�еķ�Ӧ ����ǡ����ǡ���������ԭ��Ӧ��
��2��Aװ�û���������ȡ���� ��ֻ��һ�֣���
��3��ʵ�����ռ������ķ����� ��
��4��C��Dװ������ɫ�ᷢ���仯���� ���C����D������
��5��Ϊ��ֹ����������ɿ�����Ⱦ����Ҫ������װ�õ�ĩ������һ��β������װ�ã����ʵ�װ���� ���F����G������
��6����ʵ�����һ��ʱ���ѹEװ���еĽ�ͷ�ιܣ�����1-2��Ũ���ᣬ�ɹ۲쵽�������� ��
��7��2010 �� 11��9��������ʡһ�䶳�������� ��й©�¼���500 �������ҹ��ת�ơ����������ֳ���������ʲô�Ծ�Ϊ���� ��
��й©�¼���500 �������ҹ��ת�ơ����������ֳ���������ʲô�Ծ�Ϊ���� ��
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O
��1��A�еķ�Ӧ ����ǡ����ǡ���������ԭ��Ӧ��
��2��Aװ�û���������ȡ���� ��ֻ��һ�֣���
��3��ʵ�����ռ������ķ����� ��
��4��C��Dװ������ɫ�ᷢ���仯���� ���C����D������
��5��Ϊ��ֹ����������ɿ�����Ⱦ����Ҫ������װ�õ�ĩ������һ��β������װ�ã����ʵ�װ���� ���F����G������
��6����ʵ�����һ��ʱ���ѹEװ���еĽ�ͷ�ιܣ�����1-2��Ũ���ᣬ�ɹ۲쵽�������� ��
��7��2010 �� 11��9��������ʡһ�䶳��������
 ��й©�¼���500 �������ҹ��ת�ơ����������ֳ���������ʲô�Ծ�Ϊ���� ��
��й©�¼���500 �������ҹ��ת�ơ����������ֳ���������ʲô�Ծ�Ϊ���� ���� 1������
1������
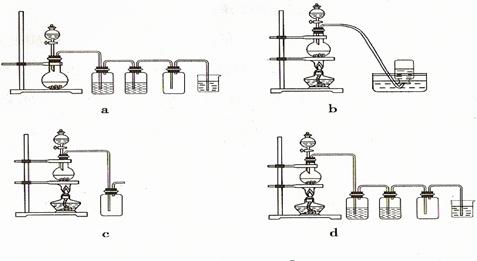
��2��O2��������������������Ҳ���֣�
��3�������ſ�����
��4��D
��5��F
��6���������
��7������ʪë����ס�ڱǣ���Ѹ�ٳ��롣�ڵ�ͷ���������ʹ��ܣ�Ѹ�ٳ��롣����籼�ܣ�Ѹ�ٳ��롣�ܴ��Ϸ�����ߣ�Ѹ�ٳ��롣��������ѡ��һ������������Ҳ���֣�
 1������
1������ ��2��O2��������������������Ҳ���֣�
��3�������ſ�����
��4��D
��5��F
��6���������
��7������ʪë����ס�ڱǣ���Ѹ�ٳ��롣�ڵ�ͷ���������ʹ��ܣ�Ѹ�ٳ��롣����籼�ܣ�Ѹ�ٳ��롣�ܴ��Ϸ�����ߣ�Ѹ�ٳ��롣��������ѡ��һ������������Ҳ���֣�
��

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ



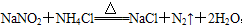 �����ڸ÷�Ӧ��˵����ȷ����(����)
�����ڸ÷�Ӧ��˵����ȷ����(����) ��3I2+3H2O+3K2SO4
��3I2+3H2O+3K2SO4