��Ŀ����
����Ŀ����þ�����������Σ��������Ʊ����ᣨH3BO3����MgO���������£���þ�����(NH4)2SO4��Һ��Ϻ���ȣ���Ӧ����H3BO3�����MgSO4��Һ��ͬʱ�ų�NH3������MgSO4��Һ��ͨ��NH3��CO2���õ�MgCO3��������Һ��������ϴ�ӡ����պ��MgO����Һ��ѭ��ʹ�á��ش��������⣺
��1������������ƣ������νṹҲ�Ƚϸ��ӣ���Ӳ���ʯ��ѧʽΪCa2B6O11��5H2O�������дΪ���������ʽ_____________________________��
��2�������Ʊ������У��������ϴ���Ƿ���ȫ�ķ�����_______________________��
��3��д��MgSO4��Һ��ͨ��NH3��CO2��Ӧ�Ļ�ѧ����ʽ_______________________��
��4����ȷ��ȡ1.68 g��þ����ȫ��Ӧ���H3BO3����1.24 g��MgO 0.8 g������������ε���ɡ���д��������̣�___________________
���𰸡�2CaO��3B2O3��5H2O ȡ�������һ��ϴ����Һ������1��2��Ba(NO3)2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ�� MgSO4+CO2+2NH3+H2O=MgCO3+(NH4)2SO4 ��þ���д��aMgObB2O3cH2O��H3BO3��������ʵ���Ϊ��![]() =0.02mol��n��B2O3��=0.01mol��n��MgO��=
=0.02mol��n��B2O3��=0.01mol��n��MgO��=![]() ="0.02" mol��n��H2O��=
="0.02" mol��n��H2O��=![]() =0.01mol��
=0.01mol��
�������Ϊ2MgOB2O3H2O��Mg2B2O5H2O��
��������
��1��Ӳ���ʯ��ѧʽΪCa2B6O115H2O�������дΪ������Ϊ��2CaO3B2O35H2O���ʴ�Ϊ2CaO3B2O35H2O��
��2��ȡ�������һ��ϴ����Һ������1��2��Ba��NO3��2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ���ʴ�Ϊȡ�������һ��ϴ����Һ������1��2��Ba��NO3��2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ��
��3��MgSO4��Һ��ͨ��NH3��CO2��Ӧ�õ�MgCO3�����ͣ�NH4��2SO4������ʽΪ��MgSO4+CO2+2NH3+H2O�TMgCO3+��NH4��2SO4���ʴ�ΪMgSO4+CO2+2NH3+H2O�TMgCO3+(NH4)2SO4��
��4����þ���д��aMgObB2O3cH2O��H3BO3��������ʵ���Ϊ��![]() =0.02mol��n��B2O3��=0.01mol��n��MgO��=
=0.02mol��n��B2O3��=0.01mol��n��MgO��=![]() =0.02 mol��n��H2O��=
=0.02 mol��n��H2O��=![]() =0.01mol��
=0.01mol��
�������Ϊ2MgOB2O3H2O��Mg2B2O5H2O���ʴ�Ϊ2MgOB2O3H2O��Mg2B2O5H2O

 ÿ��10���ӿ�����������������ϵ�д�
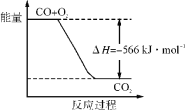
ÿ��10���ӿ�����������������ϵ�д�����Ŀ���¶�ΪTʱ����2.0 L�����ܱ������г���1.0 mol PCl5����ӦPCl5(g)===PCl3(g)��Cl2(g)����һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
����˵����ȷ����
A. ��Ӧ��ǰ50 s��ƽ������v(PCl3)��0.0032 mol��L-1��s-1
B. ���������������䣬�����¶ȣ�ƽ��ʱc(PCl3)��0.11 mol��L-1����Ӧ����H��0
C. ��ͬ�¶��£���ʼʱ�������г���1.0 mol PCl5��0.20 mol PCl3��0.20 mol Cl2����Ӧ�ﵽƽ��ǰv(��)��v(��)
D. ��ͬ�¶��£���ʼʱ�������г���2.0 mol PCl3��2.0 mol Cl2���ﵽƽ��ʱ��PCl3��ת����С��80%