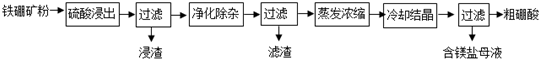
��Ŀ����
(1)��ijҩƷ����ԼΪ32.0g�� ������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�£�
50g | 20g | 20g | 10g | 5g |
(2)����500mL 0.1mol.L-1 Na2CO3��Һ��ͼ�в�������Ӧ����д������Ϊ__________��ʵ��������Ⱥ�˳��Ϊ________________ (����)��
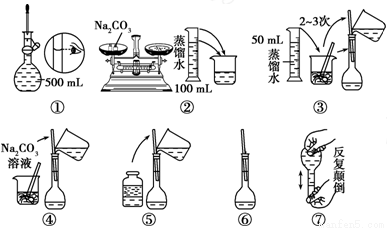
(3)������һ�����ʵ���Ũ�ȵ���Һʱ���á�ƫ�ߡ�ƫ�͡���Ӱ�족��ʾ���в�����������ҺŨ�ȵ�Ӱ�졣
������ͲȡҺ̬���ʣ�����ʱ��������Ͳ����������Һ��Ũ��___________
�ڽ���ȡҺ̬���ʵ���Ͳ��ˮϴ�ӣ�ϴ��Һ��������ƿ����������Һ��Ũ��___________
�۶���ҡ�Ⱥ���������Һ����������������Һ��Ũ��___________
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
7��4.2gijϩ����16g��ǡ����ȫ��Ӧ�����ϩ���ǣ�������
| A�� | ��ϩ | B�� | ��ϩ | C�� | ��ϩ | D�� | �κ�ϩ�� |


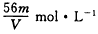 B��
B��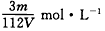 C��
C��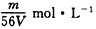 D��
D��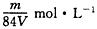
 ��Ӧ��ϵ�й����������ʣ�NH4Cl��
��Ӧ��ϵ�й����������ʣ�NH4Cl�� FeCl3��N2��Fe2O3��Fe��X��
FeCl3��N2��Fe2O3��Fe��X��