��Ŀ����
�ĸ������ͬ���ܱ���������һ���������·�����Ӧ��2SO2+O2 2SO3����Ӧ��ʼʱ����Ӧ�����ɴ�С����˳����ȷ���ǣ� ��
2SO3����Ӧ��ʼʱ����Ӧ�����ɴ�С����˳����ȷ���ǣ� ��
A����>��>��>�� B����>��>��>�� C����>��=��>�� D����>��>��=��
 2SO3����Ӧ��ʼʱ����Ӧ�����ɴ�С����˳����ȷ���ǣ� ��
2SO3����Ӧ��ʼʱ����Ӧ�����ɴ�С����˳����ȷ���ǣ� ��| ���� | �¶� | SO2(mol) | O2(mol) | ���� |
| �� | 5000C | 10 | 5 | - |
| �� | 5000C | 8 | 5 | V2O5 |
| �� | 4500C | 8 | 5 | - |
| �� | 5000C | 8 | 5 | - |
A
�¶�Խ�߷�Ӧ����Խ�죻Ũ��Խ�߷�Ӧ����Խ�죻�д�����Ӧ����Խ�죻������������ȷѡ��ΪA��

��ϰ��ϵ�д�
�����Ŀ
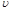 ��OE����
��OE����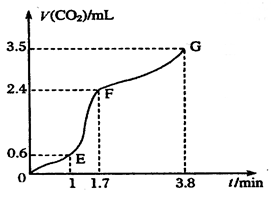
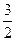 O2��g�� ��H
O2��g�� ��H =+765.2kJ��mol-1
=+765.2kJ��mol-1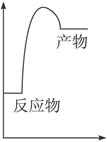
 2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60molN2��g����1.60molH2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH2�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60molN2��g����1.60molH2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH2�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ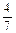 ������
������ ��
�� Ӧ������������ѧ����֪ʶ��������Ӧ���������ԭ����________________ __________________��������һ��ʱ���Ӧ�����ּ�����������ʼ�����ԭ����_______________________________��
Ӧ������������ѧ����֪ʶ��������Ӧ���������ԭ����________________ __________________��������һ��ʱ���Ӧ�����ּ�����������ʼ�����ԭ����_______________________________�� CH3COCH2I��H����I����25��ʱ���÷�Ӧ�����������о��鹫ʽ������
CH3COCH2I��H����I����25��ʱ���÷�Ӧ�����������о��鹫ʽ������ pC(g)��
pC(g)��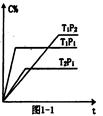
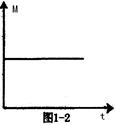
 ����
����