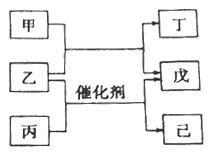
��Ŀ����
����Ŀ����11�֣�����H�׳��¿��ᣬ���Ʊ���Чճ�ϼ��ȶ��־�ϸ��ѧƷ����Ҫԭ�ϣ��ɾ����з�Ӧ·�ߵõ���

�ش��������⣺
��1��C4H8�����ƣ�ϵͳ�������� ��
��2����Ӧ���Ļ�ѧ����ʽΪ ��
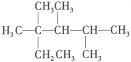
��3���¿����ж���ͬ���칹�壬�������¿�������ͬ���Һ˴Ź���������5������л���ṹ��ʽΪ ��
��4�����й���H��˵����ȷ���� ������ĸ��
a����ʹ����KMnO4��Һ�����CCl4��Һ��ɫ
b������Na2CO3��Ӧ��������HBr��Ӧ
c����������Cu(OH)2��Ӧ
d��1molH��ȫȼ������5molO2
���𰸡���11�֣�
��1��2������1����ϩ��2�֣�
��2��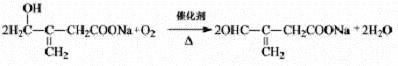 ��2�֣�
��2�֣�
��3��HOOCCH=CHCH3COOH��2�֣�
��4��a��c��2�֣�
�������������������1�����ݲ���Ľṹ����֪�л����̼����2��̼��һ����������C4H8�������ǣ�2������1����ϩ��
��2����Ӧ��Ϊ��Ӧ���е��ǻ�������Ϊȩ���ķ�Ӧ�����Ի�ѧ����ʽΪ��
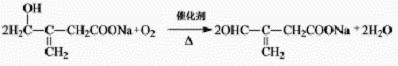
��3�����¿�������ͬ�࣬���������ͬ�������Ȼ���̼̼˫�����˴Ź���������5���壬˵��Hԭ�ӵ�λ����5�֣����Ը����ʵĽṹ��ʽΪ��HOOCCH=CHCH3COOH��
��4��a��H����̼̼˫����������ʹ����KMnO4��Һ�����CCl4��Һ��ɫ����a��ȷ��b��H����̼̼˫��������HBr�����ӳɷ�Ӧ����b����c��H�����Ȼ�����������Cu(OH)2�����кͷ�Ӧ����c��ȷ��d��H�ķ���ʽΪC5H6O4������1mol H��ȫȼ������4.5mol O2����d����

����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CH4(g)+H2O(g)![]() CO(g)+3H2(g)����ͬ�¶��´ﵽƽ��ʱ����Ӧ��ϵ�и����ʵ�Ũ�����±���
CO(g)+3H2(g)����ͬ�¶��´ﵽƽ��ʱ����Ӧ��ϵ�и����ʵ�Ũ�����±���
�¶� | CH4(g) | H2O(g) | CO(g) | H2(g) |
500K | 0.8mol/L | 0.8mol/L | 0.2mol/L | 0.6mol/L |
800K | 0.6mol/L | 0.6mol/L | 0.4mol/L | 1.2mol/L |
1000K | 0.4mol/L | 0.4mol/L | 0.6mol/L | 1.8mol/L |
(1)�÷�Ӧ��ƽ�ⳣ������ʽΪK=______________������ӦΪ_________��Ӧ������ȡ����ȣ���
(2)���жϸ÷�Ӧ�Ƿ��Ѵﵽ��ѧƽ��״̬��������_________
a������ѹǿ���� b��c(CH4)=c(H2O)
c��v��(CH4)=v��(H2) d �����������c(CO)����
�������ǹ��ۼ��������̼�Ȼ�ԭ�Ʊ����������ܷ�Ӧ��ѧ����ʽΪ��δ��ƽ����
Al2O3(s)+C(s)+N2(g)��AlN(s)+CO(g) ������
ij��������Ʒ�к������������ʣ���֪��������NaOH��Һ�ܷ�Ӧ���������������䷴Ӧ����ʽΪ��AlN+NaOH+H2O��NaAlO2+NH3�������ڣ�
������Ҳ��������������Һ��Ӧ��ȫ���ܽ⡭����
(3)��ƽ��ѧ��ѧ����ʽ�٣�___Al2O3(s)+ C(s)+ N2(g)�� AlN(s)+ CO(g)
(4)��Ӧ���в�������ļ��鷽��Ϊ____________________________________________��
��Ӧ�۵����ӷ���ʽ___________________________________________________________��