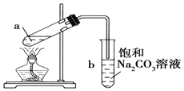
��Ŀ����
����Ŀ��ʵ���ҿ����Ҵ��Ʊ������飺CH3CH2OH+HBr![]() CH3CH2Br+H2O����ͨ�������廯�ƺ�Ũ�������Ҵ����ȵķ�����������ijͬѧ�Ը�ʵ��IJ��룬����Ϊ���ܴ�����ǣ� ��
CH3CH2Br+H2O����ͨ�������廯�ƺ�Ũ�������Ҵ����ȵķ�����������ijͬѧ�Ը�ʵ��IJ��룬����Ϊ���ܴ�����ǣ� ��
A.�Ʊ������п��ܿ��������ݲ���
B.���Խ�������NaBr��H2SO4(Ũ![]() ��CH3CH2OH����ҩƷ����һ�������м����Ʊ�CH3CH2Br
��CH3CH2OH����ҩƷ����һ�������м����Ʊ�CH3CH2Br
C.�����������Ʊ�CH3CH2Br��ʵ�飬ʵ���ռ����IJ�����ܽ���
D.Ϊ�˳�ȥCH3CH2Br�л��е�HBr�������ȵ�NaOH��Һϴ��
���𰸡�D
��������
A.�Ʊ�CH3CH2Br�����У�CH3CH2OH���ܷ�����ȥ��Ӧ����CH2=CH2������������ݣ�A����ȷ��
B.Ϊ����ҩƷ��ĺͲ����ɽ�������NaBr��H2SO4![]() Ũ
Ũ![]() ��CH3CH2OH����ҩƷ����һ�������м��ȷ�Ӧ��B����ȷ��
��CH3CH2OH����ҩƷ����һ�������м��ȷ�Ӧ��B����ȷ��
C.�������ɵ�CH3CH2Br�е�ϵͣ��ļ���������ʹ��Ʒ�ӷ�������ʵ���ռ����IJ�����٣�C����ȷ��
D.CH3CH2Br���ȵ�NaOH��Һ�лᷢ��ˮ�ⷴӦ��D�����

 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�����Ŀ���ظ����[(NH4)2Cr2O7]���������Լ���������ýȾ���ȡ�ʵ���ҳ����ü�ȩ���ⶨ�ظ������Ʒ�е��������������䷴Ӧԭ��Ϊ2Ba2++Cr2O72+H2O = 2BaCrO4��+2H+��4NH4++6HCHO = 3H++6H2O+(CH2)6N4H+ [�ζ�ʱ��1 mol (CH2)6N4H+��1 mol H+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣ
ʵ�鲽�裺��ȡ��Ʒ2.800 g�����250 mL��Һ����ȡ25.00 mL��Ʒ��Һ����ƿ�У��������ᱵ��ҺʹCr2O72��ȫ��������10 mL 20%�����Լ�ȩ��Һ��ҡ�ȣ�����5 min����1~2�η�̪��Һ����0.200 mol��L-1 NaOH����Һ�ζ����յ㣬�ظ���������3�Ρ��ζ�������±���ʾ��
�ζ����� | ������Һ�����/mL | ����Һ����� | |
�ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
1 | 25.00 | 1.05 | 21.06 |
2 | 25.00 | 1.98 | 21.97 |
3 | 25.00 | 0.20 | 21.98 |
����Ʒ�е�����������Ϊ_________(���������λС��)����д����Ҫ��������̡�
����Ŀ������ʯ����Ҫ�ɷ�ΪBaCO3(��Ca2+��Mg2+��Fe3+������)��ʵ�������ö���ʯ�Ʊ�BaCl2��2H2O���������£�

Ca2+ | Mg2+ | Fe3+ | |
��ʼ����ʱ��pH | 11.9 | 9.1 | 1.9 |
��ȫ����ʱ��pH | 13.9 | 11.1 | 3.2 |
(1)����ʯ�������ȡǰ������ĥ��Ŀ����________��
(2)ʵ������37%����������15%�����ᣬ����Ͳ���ʹ�����������е�________________��
A.�ձ� B.����ƿ C.������ D.�ζ���
(3)����NH3��H2O��pH=8�ɳ�ȥ________________(�����ӷ���)���������к�________________(�ѧʽ)��
(4)���ü�����ζ����ɲⶨBa2+�ĺ�����ʵ����������С�
��֪��2CrO42-+2H+![]() Cr2O72-+2H2O Ba2++CrO42-=BaCrO4��
Cr2O72-+2H2O Ba2++CrO42-=BaCrO4��
�������ȡx mLһ��Ũ�ȵ�Na2CrO4��Һ����ƿ�У��������ָʾ������b mol��L-1�����Һ�ζ����յ㣬��õμ���������ΪV0 mL��
�������ȡy mL BaCl2��Һ����ƿ�У�����x mL�벽�����ͬŨ�ȵ�Na2CrO4��Һ����Ba2+��ȫ�������ټ������ָʾ������b mol��L-1�����Һ�ζ����յ㣬��õμ���������ΪV1 mL��
��BaCl2��Һ��Ũ��Ϊ________________mol��L-1��
����������еμ�����ʱ����������Һ������Ba2+Ũ�Ȳ���ֵ��____________(����ƫ��������ƫС��)��
����Ŀ������ѪҺ�������Ҫ�����ƽ�⣺![]() ��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��
��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��![]() �仯��ϵ���±���
�仯��ϵ���±���
| 1.0 | 17.8 | 20.0 | 22.4 |
pH | 6.10 | 7.35 | 7.40 | 7.45 |
����˵������ȷ����
A. ��������ѪҺ�У� ![]() ��ˮ��̶ȴ��ڵ���̶�
��ˮ��̶ȴ��ڵ���̶�
B. ����ѪҺ���ж�ʱ����ע��![]() ��Һ����
��Һ����
C. ![]() ��ѪҺ�У�
��ѪҺ�У� ![]()
D. pH=7.40��ѪҺ�У� ![]() ��ˮ��̶�һ������
��ˮ��̶�һ������![]() �ĵ���̶�
�ĵ���̶�