��Ŀ����
����Ŀ���������ֳ����£�N2H4����ɫҺ�壩��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ����������ȼ�ϣ��ش��������⣺
��1���������ӵĵ���ʽΪ__________�����е��Ļ��ϼ�Ϊ______��
��2��ʵ���ҿ��ô���������Һ�백��Ӧ�Ʊ���������Ӧ�����ӷ���ʽ____________��
��3��������N2O4����Ϊ����ƽ������䷴Ӧ�IJ��������Ⱦ������������ͻ�ԭ����֮��Ϊ _______��
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽��ʽ�백���ơ������������γɵ���ʽ�εĻ�ѧʽΪ______________��
���𰸡�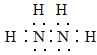 -2 2NH3+NaClO=N2H4+ NaCl+ H2O 2:1 N2H6(HSO4)2
-2 2NH3+NaClO=N2H4+ NaCl+ H2O 2:1 N2H6(HSO4)2
��������
��1����������ʽΪN2H4����ԭ���γ�3������������ʽΪ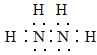 �����ݻ��ϼ۴����͵���0��������Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�Ϊ-2�ۡ�
�����ݻ��ϼ۴����͵���0��������Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�Ϊ-2�ۡ�
��2���������ưѰ�������Ϊ�������������Ʊ���ԭΪ�Ȼ��ƣ����ݵ�ʧ�����غ㣬��Ӧ�ķ���ʽ��2NH3+NaClO=N2H4+ NaCl+ H2O��
��3��������N2O4����Ϊ����ƽ�������Ӧ�IJ���Ϊ����Ⱦ�ĵ�����ˮ��N2O4��NԪ�ػ��ϼ���+4����Ϊ0��N2H4��NԪ�ػ��ϼ���-2����Ϊ0�����ݵ�ʧ�����غ㣬����������ͻ�ԭ����֮��Ϊ2:1��
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽��ʽ�백���ƣ��γ�������ΪN2H62+���������γɵ���ʽ�εĻ�ѧʽΪN2H6(HSO4)2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�