��Ŀ����
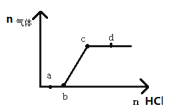
����Ŀ��25��ʱ������(HM)������(NaM)��ɵĻ����Һ����ʼŨ�Ⱦ�Ϊlmol��L-1�������Һ��ͨ��HC1��������NaOH����ʱ����ҺpH�ı仯������ͼ��ʾ������˵������ȷ����

A. C��ʱ����Һ�� c(Na+)=c(M-)
B. �� D��E ����Һ��Ϻ�c(M-)+c(HM)=2c(Na+)
C. B��ʱ����Һ�� c(M-)>c(Na+)>c(HM)
D. A��B��C��������ʾ����Һ��ˮ���������c(H+)������
���𰸡�B
�����������ݵ���غ㣬C����Һ�����ԣ���Һ�� c(Na+)=c(M-)����A��ȷ��
���������غ㣬B�����Һ��c(M-)+c(HM)=2c(Na+)���� D��E ����Һ��Ϻ����������࣬��B����B����Һ�����ԣ�HM�������NaMˮ�⣬��Һ�� c(M-)>c(Na+)>c(HM)����C��ȷ��A����B����Һ�м����ᣬ��ˮ�������Ƴ̶�����C������B����Һ�м��������ƣ�����HM��ˮ�ĵ���̶���������A��B��C��������ʾ����Һ��ˮ���������c(H+)����������D��ȷ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������X����(��ͨ��)Y��Һ��,���ɳ������������X�����ʵ�����ϵ��ͼ��ʾ,����ͼʾ�������( )

A | B | C | D | |
X | CO2 | HCl | NaOH | AlCl3 |
Y | Ca(OH)2 | NaAlO2 | AlCl3 | NaOH |
A. A B. B C. C D. D