��Ŀ����
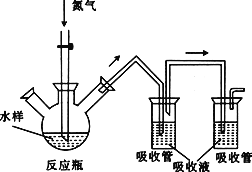
����Ŀ��ʵ��������ͼ��ʾ��ʵ��װ�ã�
A C
C D
D E
E ![]() F
F
��1����֪������NH3����һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С����������ˮ����ʵ������ͨ���ü��ȹ����Ȼ�狀��������ƵĻ��������ȡ�������Իش��������⣺
��ʵ������ȡ��������ѡ��________װ�ã��ռ���������ѡ��________װ�á�
����д��ʵ������ȡ�����Ļ�ѧ����ʽ��____________________________________��
����μ����ռ��������ǰ�������д�������ͽ��ۣ�__________________________��
��2���ס�������ͬѧ�ø����Բ����ƿ���ռ�һƿ����������ͼB��Ȫʵ���װ�ý���ʵ�飬���۲쵽�����ĺ�ɫ��Ȫ��
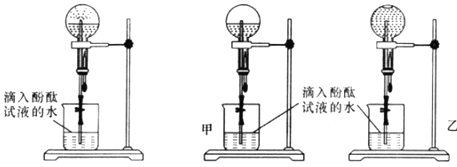
ͼB����Ȫʵ��װ�� ͼC������ʵ�����Ա�
����ʵ������˵���������е�������___________��____________��
��3���ס�������ͬѧ�����Ȫʵ���Բ����ƿ��������Һ��ͼC��ʾ����ͨ������ȷ�ϣ�����ͬѧ���ð�ˮ�����ʵ���Ũ��_______����������������С��������������������ͬѧ���ð�ˮ�����ʵ���Ũ�ȡ�
���𰸡�A E 2NH4Cl+Ca(OH)2![]() 2NH3��+2H2O+CaCl2 ��պ��Ũ����IJ����������Թܿڣ�����������̣���֤��Ϊ���� ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ��������֤���ǰ��� ��������ˮ������ˮ����Һ�ʼ��� ����
2NH3��+2H2O+CaCl2 ��պ��Ũ����IJ����������Թܿڣ�����������̣���֤��Ϊ���� ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ��������֤���ǰ��� ��������ˮ������ˮ����Һ�ʼ��� ����
��������
(1)��ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ�Ʊ�����������������ˮ���ܶȱȿ���С��
���������ƺ��Ȼ���ڼ������������ɰ������Ȼ��ƣ�
�۰���Ϊ�������壬��ˮ��Ӧ����NH3H2O�������OH-���ӣ���Һ�ʼ��ԣ���������ʪ��ĺ�ɫʯ����ֽ���飬Ҳ�������ð������Ȼ��������������ɰ������ʵ�飻
(2)��ɫ��̪��Һ������ɫ����������ˮ���������γ���Ȫ��
(3)������������ˮ��������ƿ��Һ�����������ĵİ��������ȡ�
(1)��ʵ������ȡ���������ù����ϼ��ȣ���ѡ��Aװ�ã�����������ˮ���ܶȱȿ���С���ռ�����ֻ��ѡ�������ſ������ռ�������ѡ��Eװ�ã�
��ʵ�������������ƺ��Ȼ���ڼ����������Ʊ���������Ӧ����ʽΪ2NH4Cl+Ca(OH)2![]() 2NH3��+2H2O+CaCl2��
2NH3��+2H2O+CaCl2��
�۰���Ϊ�������壬��ˮ��Ӧ����NH3H2O�������OH-���ӣ���Һ�ʼ��ԣ�����ʱ������պ��Ũ����IJ����������Թܿڣ�����������̣���֤��Ϊ����������ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ��������֤���ǰ���������
(2)��ɫ��̪������ɫ������ͼ2��Ȫʵ���װ�ý���ʵ�飬���۲쵽�����ĺ�ɫ��Ȫ��������ˮ��Һ�ʼ��ԣ�������������ˮ���γ�ѹǿ������γ���Ȫ��
(3)������������ˮ��������ƿ��Һ�����������ĵİ��������ȣ���ˮ�����ʵ���Ũ��Ϊ=![]() =
=![]() =
=![]() ���백��������أ���˼���ͬѧ���ð�ˮ�����ʵ���Ũ�ȵ�������ͬѧ���ð�ˮ�����ʵ���Ũ�ȡ�
���백��������أ���˼���ͬѧ���ð�ˮ�����ʵ���Ũ�ȵ�������ͬѧ���ð�ˮ�����ʵ���Ũ�ȡ�