��Ŀ����
��������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�ã�
��1����������Ӧ��ʵ���У����ᡢ�Ҵ���������ƽ��ʱ�������������������±���
�ɱ��������Ʋ⣬��ֵx�ķ�Χ��______��
��2������25��ʱ��pH=3�Ĵ��ᣮ��ش��������⣺
����������м������������ƹ��壬��ʱ��Һ��
����______�����������С�����䡱����
����������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������Һ��pH______7�����������������=������
����������м���pH=11��NaOH��Һ���Ҷ��ߵ������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����
______��
��1����������Ӧ��ʵ���У����ᡢ�Ҵ���������ƽ��ʱ�������������������±���
| ��Ӧ | �Ҵ���mol�� | ���ᣨmol�� | ����������mol�� |
| 1 | 2 | 2 | 1.33 |
| 2 | 3 | 2 | 1.57 |
| 3 | 4 | 2 | x |
| 4 | 5 | 2 | 1.76 |
��2������25��ʱ��pH=3�Ĵ��ᣮ��ش��������⣺
����������м������������ƹ��壬��ʱ��Һ��
| c(H+) |
| c(CH3COOH) |
����������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������Һ��pH______7�����������������=������
����������м���pH=11��NaOH��Һ���Ҷ��ߵ������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����
______��
��1��������������ʵ�����ͬ�����£������Ҵ������ʵ���ƽ�������ƶ����������������ʵ������ӣ������Ҵ������ʵ���ƽ�������ƶ����������������ʵ������٣�����1.57��X��1.76���ʴ�Ϊ��1.57��X��1.76��
��2����������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���C��H+����С��
C��CH3COOH����������
��С���ʴ�Ϊ����С��
��������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ�������˴����ƣ�������ˮ��ʼ��ԣ��ʴ�Ϊ������
�۴����������Һ�еijɷ�Ϊ����ʹ����ƣ������ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ�����c��CH3COO-����
c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
��2����������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���C��H+����С��
C��CH3COOH����������
| C(H+) |
| C(CH3COOH) |
��������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ�������˴����ƣ�������ˮ��ʼ��ԣ��ʴ�Ϊ������
�۴����������Һ�еijɷ�Ϊ����ʹ����ƣ������ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ�����c��CH3COO-����
c��Na+����c��H+����c��OH-�����ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
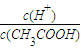 ��______�����������С�����䡱����
��______�����������С�����䡱����