��Ŀ����
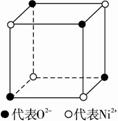
��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������塣NiO��������������Ľṹ��NaCl��ͬ��Ni2+�����ڽ�O2���ĺ˼����Ϊa��10��8 cm������NiO������ܶȡ�����֪NiO��Ħ������Ϊ74.7 g��mol��1��
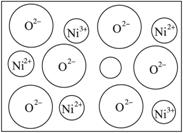
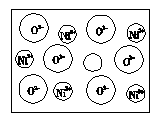
��2����Ȼ�ĺ;����˹��Ʊ��ľ��嶼����ȱ�ݣ�������ij��NiO�����оʹ�����ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ���������Ǿ����Գʵ����ԣ�����������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ���ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�
��������1��1 cm3����������������=��![]() ��3
��3
1 cm3��Ni2+��O2�����Ӷ���=��![]() ��3��
��3��![]()
�ܶ�=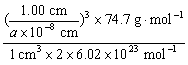 =
=![]() g��cm��3��
g��cm��3��
��2����С��ⷨ�ж��֣�������ܼ��ַ���
��һ������غ㷨
��1����Ni3+�����ʵ���Ϊx��Ni2+�����ʵ���Ϊ��0.97��x����
3x+2����0.97��x��=2��1
x=0.06 mol ![]() =
=![]() ��
��
��2����![]() =
=![]() ����1 mol Ni0.97O��
����1 mol Ni0.97O��
��Ni3+��![]() ��0.97 mol��Ni2+��
��0.97 mol��Ni2+��![]() ��0.97 mol��
��0.97 mol��
![]() ��0.97 mol��3+
��0.97 mol��3+![]() ��0.97 mol��2=2��1 mol
��0.97 mol��2=2��1 mol
![]() =
=![]() ��
��
�������з����飨ʽ����
��3������a��Ni3+��b��Ni2+��c��O2����
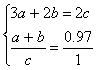 ���
���![]() =
=![]()
��4����NiO���ʵ���Ϊx��Ni2O3���ʵ���Ϊy��
![]() ���
���![]()
![]() =
=![]() ��
��
��5����Ni��Oԭ�Ӹ���֮�ȡ�

![]() =
=![]() =
=![]() ���
���![]() =
=![]() ��
��
������ʮ�ֽ��淨
��6�����ϼ۵�ʮ�ֽ��棬��Ni0.97O��Ni���ϼ�Ϊ![]() ��
��

![]() =
=![]() =
=![]() ��
��
��7�������ʮ�ֽ��档
��Ni2O3��![]() O
O
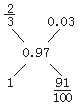
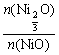 =
=![]()
![]() =
=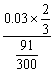 =
=![]() ��
��
���ģ�����������
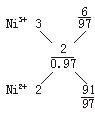
��8��100����ԭ�Ӷ�Ӧ97��Niԭ�ӣ���Ni2+��Ni3+����1�����ۣ�ȱ1��Ni2+��ʹ2��Ni2+��ΪNi3+����ô��ȱ3��Ni2+������6��Ni2+��ΪNi3+��
![]() =
=![]() =
=![]() ��
��
�𰸣�6��91

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�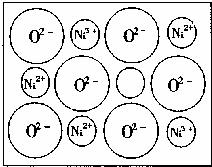 ��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������壮 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7g��mol-1����
��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������壮 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7g��mol-1����