��Ŀ����
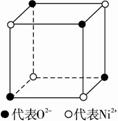
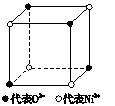
��1����ѧ�̲���ͼʾ��NaCl����Ľṹ��������ά�ռ������γ��������塣��������NiO������Ľṹ��NaCl��ͬ��Ni2+�����ڽ���O2-�˼����Ϊa��10-8 cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7 g�� mol-1����
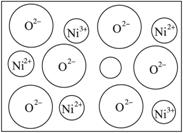
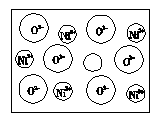
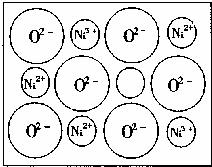
��2����Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�������ͼ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ�����������Ni��O�ĸ�����ȴ�����˱仯��ij��������Ʒ�����ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�
��1��![]() g��cm-3 ��2��6��91
g��cm-3 ��2��6��91
����:
��1��ѡ�����µ�һ��С�����������м��㣺
��ͳ�ƵĽǶȿ�����С��������е�Ni2+��O2-���Ӷ���Ϊ0.5��
�������������![]() ��0.5=6.20��10-23 g
��0.5=6.20��10-23 g
��������������a��10-8 cm )3=a3��10-24 cm3
С��������ܶ�Ϊ��![]() =
=![]()
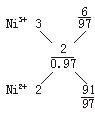
��2����1 mol Ni0.97O�к�Ni3+�����ʵ���Ϊx����Ni2+�����ʵ���Ϊ0.97��x��
���ݾ���ʵ����Կ��г���3x+2(0.97��x)=2�����x=0.06
��������֮��Ϊ��Ni3+��Ni2+=0.06��(0.97��0.06)=6��91

 ��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������壮 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7g��mol-1����
��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������壮 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7g��mol-1����